|
|
1.IntroductionBulk heterojunction (BHJ) organic solar cells are currently in the focus of interest because they offer a promising approach toward low-cost regenerative energy sources.1, 2 They are flexible, light weight, and can be deposited from solution (e.g., by a printing process using role-to-role fabrication technology) and have shown a power conversion efficiency (PCE) of up to 7.4%.3, 4 , 5 The active layer of BHJ solar cells consists of a bicontinous interpenetrating network of electron donor and acceptor domains, which is formed during the deposition/drying process. Currently, the favored materials for BHJ solar cells are composites of fullerenes such as [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) as electron acceptor materials with semiconducting polymers, such as P3HT or PCPDTBT as electron donor materials. In order to fully exploit the potential of BHJ solar cells, the search for new donor materials is ongoing.6 Soluble small molecules are attractive candidates for solution-processed BHJ solar cells, because they offer the same processing advantages commonly associated with polymers, but they are easier to synthesize and, above all, to purify. In addition, contrary to polymers, small molecules do not suffer from polydispersity, whereas the performance of polymeric solar cells is often affected by the molecular weight and the weight distribution of the respective material batch.7 Recently, the first BHJ solar cells that are based entirely on small conjugated molecules (i.e., PCBM as electron acceptor and various electron donor components) have been published, indicating the increasing interest in this emerging research field.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 With dendrimers or oligomers based on typical organic semiconductors derived from oligothiophenes, PCE values up to 2.5% could be achieved.10, 11 , 12 Comparable values were also reported for pigment-based solar cells using acene,13, 14 , 15 squaraine,16, 17 boron dipyrromethene,18 and merocyanine dyes.19 Recently, outstanding PCE values of up to 4.4% have been reported by Tamayo20 and Walker21 for molecules composed of two subunits, a dye and a semiconductor part. In our recent work, we have introduced traditional colorants, in particular, merocyanine (MC) dyes that are widely applied in textile coloration, printing applications, and nonlinear optics, as a new class of donor material for organic solar cells.19 Because MCs consist of an electron-withdrawing and electron-donating subunit, connected through a π-conjugated bridge, they are rather polar compounds featuring relatively large dipole moments, which is generally considered to be disadvantageous due to limited charge-transport properties. On the other hand, in particular, if their electronic structure is in the cyanine limit (i.e., both neutral and charge-separated electronic structures contribute equally to the ground and excited state), then the optical transition dipole moment is strongly enhanced, resulting in large absorption coefficients up to 1.5×105 M−1 cm−1, which is favorable for the absorption of light and thus for the efficiency of the solar cell. The absorption of MC dyes can be easily tuned from the UV to the near-infrared by attaching substituents with various electron-withdrawing and -donating strengths.22 Therefore, the whole spectral range of the solar irradiation can be covered. Additionally, the easily tunable electrical, optical, and bulk properties of MC dyes by chemical modifications offer a new research route to a systematic study of the influences of these parameters on the solar cell performance. In this paper, we report our efforts to improve BHJ solar cells containing the MC dye MD304 (for chemical structure, see inset in Fig. 2). With a maximal absorption at 650 nm [ultraviolet-visible (UV-vis) spectrum in Fig. 1], MD304 appears deep blue. Fig. 1EQE of solar cells PEDOT/MD304:PCBM 30:70 wt% (60 nm)/Al (squares, left axis) and UV-vis spectrum of the solar cell (line, right axis). 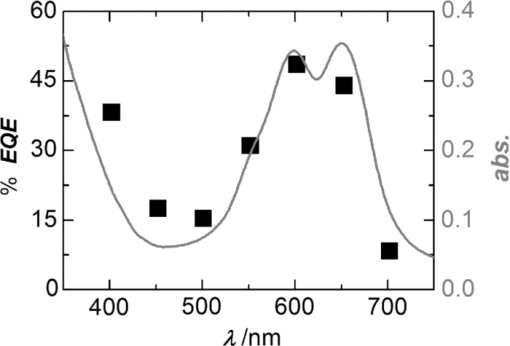 Fig. 2Illumination intensity dependence of MD304:PCBM solar cells (70 wt% PCBM, Ba/Ag cathode). (a) VOC (filled circles, left axis) and FF (open diamonds, right axis) and (b) JSC (closed squares, left axis) and PCE (open triangles, right axis). The inset in (a) shows the chemical structure of MD304 (Ph = phenyl group). 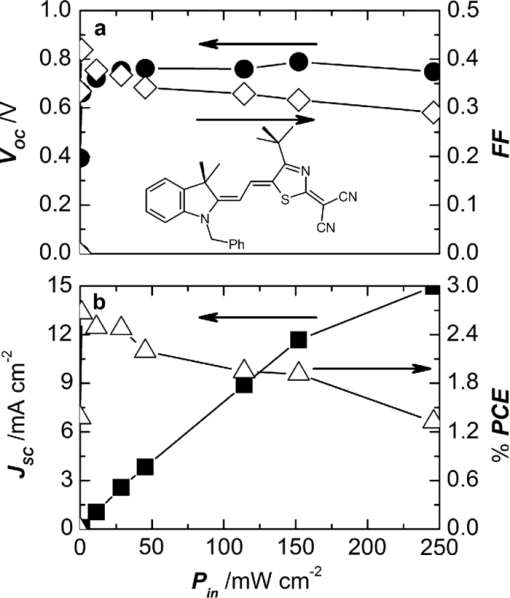 2.Results and Discussion2.1.Active Layer Thickness and Donor: Acceptor RatioIn line with our earlier work, a first series of devices with Al top electrode was fabricated. The MD304:PCBM ratio and the active layer thickness were varied, respectively. The largest PCE value of 1.74% was achieved for a PCBM content of ∼70 wt% and for rather small active layer thicknesses of ca. 60 nm; a second maximum with reduced efficiency was found for thicker active layers (ca. 170 nm).10 The optimized device features a short-circuit current (JSC) of 6.3 mA cm−2, an open-circuit voltage (VOC) of 0.76 V, and a fill factor (FF) of 0.36 (Table 1). Many solution-processed small molecule BHJ solar cells appear to feature smaller FF values compared to polymeric solar cells mostly in the range between 0.3 and 0.5.8, 9, 10, 11, 12, 13, 15, 16, 17, 18, 19, 20, 21 The highest FF reported for a solution-processed BHJ solar cell containing small molecule donors is 0.61,14 while for polymer FF >0.7 have been reported. Table 1Performance of the investigated MD304:PCBM solar cells with varying top-electrode metals (70 wt% PCBM; d ≈ 60 nm) for AM 1.5 light of 100 mW cm−2 intensity.
As is commonly done for polymer-based devices,23 we have tried to improve the device performance by adding a number of “secondary solvents” (anisole, nitrobenzene, or tetrahydrofuran) to the chlorobenzene:pyridine mixture; however, this did not enhance the device performance. Also, the commonly used curing protocols, such as heat treatment, solvent soaking, etc., did barely change the device performance. Thus, it can be assumed that the layer morphology obtained from wet deposition of small-molecule inks is much closer to thermodynamic equilibrium than for polymers, where strong annealing effects are commonly observed. 2.2.OFET Hole Mobility MeasurementsConsidering the small FF values and the facts that the maximum efficiency is achieved for a rather high PCBM content, at a rather small active layer thickness, and that the efficiency is decreased for thicker active layers (even in the second maximum), the investigated MC:PCBM solar cells seem to suffer from a poor or unbalanced charge-carrier transport. Organic field-transister measurements reveal hole mobilities in the range of 10−6 cm2 V−1 s−1 for layers of pure MD304. For mixed MD304:PCBM layers, the hole mobility decreases by roughly one order of magnitude (5×10−8 cm2 V−1 s−1 at 70 wt% PCBM). These hole mobilities are considerably smaller compared to those obtained for conjugated polymers (e.g., P3HT), measured under the identical conditions and far beyond the electron mobility of PCBM.24 This drawback might be due to the dipolar character of the dyes and could furthermore depend on unfavorable aggregation of the small molecules in the bulk.25 However, the measured hole mobilities of the MC:PCBM devices are still in the range of those obtained for α,α-DH6TDPP:PCBM solar cells (measured in hole-only devices via space-charge-limited current), which are among the most efficient small-molecule solution-processed BHJ solar cells reported thus far.20 Thus, obviously, a deficient carrier mobility is not necessarily detrimental for the solar cell performance. 2.3.EQE MeasurementsThis view is further supported by our measurements of the external quantum efficiency (EQE) in our devices (Fig. 1). The data points roughly follow the absorption spectrum of the mixture. At the wavelength of maximum absorption, EQE is ca. 50%, a value that exceeds the EQE of many other solution-processed small-molecule BHJ solar cells, which were reported to be in the range of 30–45%;10, 11, 16, 17, 18, 20 values up to 58% were also reported for devices containing a diketopyrrolopyrrole derivative.21 2.4.PermittivityThe origin behind this apparent discrepancy between a decent EQE and a rather poor carrier mobility could be the strong polarity of the MD304 (MC dyes, in general), the molecular dipole moment being ∼14 D.26 Organic materials commonly feature rather low dielectric constants (ɛr = 2–3), and as a result, the Coulomb interaction between charges is strong, leading to losses due to recombination. A more polar environment would stabilize charges and thus reduce recombination effects. Thus, we determined the permittivity of our materials by impedance spectroscopy (measurement of the devices’ capacity). For a device PEDOT:PSS/MD304 (47 nm)/Al, an ɛr of 3.4 was obtained. This compares to ɛr = 2.2 for a heat-treated (i.e., fully aggregated) layer of P3HT. Because ɛr of PCBM27 is higher than for MD304 and P3HT, the ɛr values of the actual solar cells are higher than for the neat donor compounds. For blends with optimized donor:acceptor ratios, ɛr of 4.0 for MD304 with 70 wt% PCBM and 3.6 for P3HT with 40 wt% PCBM were measured. Thus, the exciton binding energy in the MD304 layer is 0.04 eV (10%) smaller than in the P3HT blend (assuming a distance between the charges of 1 nm). It has been shown that in organic solar cells containing materials with high ɛ values, the charge dissociation in the active layer as well as the initial separation distance and the decay rate of the bound electron-hole pairs are improved.28 Overall, assuming similar mobility the performance of the solar cell is enhanced. 2.5.Variation of the Top ElectrodeIn order to further optimize the devices, we investigated the influence of the nature of the top electrode (120 nm Al, 6 nm Ca/120 nm Ag, and 6 nm Ba/120 nm Ag) on the solar cell performance (see Table 1). As expected for cathode materials with work functions above the LUMO lowest accepted molecular orbital level of PCBM,29 all cells exhibit similar values for VOC, whereas JSC increases with increasing work function ϕw of the used metal.30 This might be attributed to a higher built-in voltage (Vbi) in these devices in consequence of the increasing energetic difference between the two electrodes. However, also the different reflection properties of the metals and the resulting electric field distribution inside the active layer have influences on JSC. The best PCE value of 2.1% under AM1.5 conditions was achieved by using a Ba/Ag cathode. 2.6.Performance at Reduced Illumination IntensityThe dependency of VOC, JSC, FF, and PCE on the power of incident light (Pin) for the optimized MD304 devices is depicted in Fig. 2. Except for high intensities (>150 mW cm−2), JSC increases linearly with Pin. VOC is nearly constant for the entire intensity range and only drops strongly for light intensities near zero, in agreement with theoretical expectations. Finally, the FF increases with decreasing Pin, which is indicative of reduced recombination effects at low charge carrier concentration (low intensity), again an outcome of the poor carrier mobility. Overall, the efficiency is increased under lower light intensity, reaching 2.7% at an intensity of ∼2 mW cm−2. 3.ExperimentalThe solar cells were fabricated according to the previously reported procedure.19 For spin-coating, the dye was dissolved in a chlorobenzene:pyridine (5:1) mixture. The JV characteristics of the solar cells were measured using a Keithley 2425 source measurement unit. The AM1.5 light was provided by a filtered Xe lamp. The intensity of 100 mW cm−2 of the AM1.5 light was determined using a calibrated inorganic solar cell from the Fraunhofer Institute for Solar Research in Freiburg (Germany) and a reference PCBM:P3HT cell measured by the same institution. No mismatch factor was included in the calculation of the efficiency. EQE measurements were performed by filtering the Xe lamp using Melles Griot interference filters with an FWHM of 10 nm. For mobility measurements, transistors were fabricated by spin-coating the materials on heavily doped p-type Si++/SiOx substrates with patterned source and drain gold contacts. The doped silicon substrate was used as the common gate contact followed by a 230-nm thick SiOx insulating layer with a capacitance of 15 nF cm−2. Channel length and width were 2.5 μm and 1 cm, respectively. Devices were measured in a dry-nitrogen atmosphere with an Agilent B1500A semiconductor device analyzer. Mobilities were calculated from the transconductance in the linear regime. The dielectric constants were determined by capacity measurements using an oscillating voltage of 50 mV and a frequency between 20 Hz and 10 kHz. 4.ConclusionsMCs are very promising electron-donor compounds for organic BHJ solar cells using PCBM as the acceptor. By optimizing the cell parameters, an EQE of ca. 50% and a PCE value of 2.1% were obtained. At reduced illumination intensity (2 mW cm−2), the PCE value further increases up to 2.7%. Our data indicate that the strongly dipolar character of the MC dyes has positive, but also some negative, effects on the solar cell performance. On the one hand, the hole mobility is quite low, which might be caused by the strong energetic disorder;31 on the other hand however, charge generation is enhanced and recombination reduced due to the high dielectric permittivity of the material. Optimization of this trade-off by ideally packed MC dyes may accordingly open an avenue toward high-performance BHJ cells. In this regard, it is noteworthy that the active layer thicknesses of our best cells were only ∼60 nm (i.e., the incident light is not fully absorbed). For comparison, solar cells based on the donor polymers P3HT and OC1C10-PPV with comparable layer thickness exhibit PCE values smaller than 2%.32 By improving the hole mobility of the MC dyes, solar cells with larger optimal layer thickness and thus higher efficiencies should be achievable. AcknowledgmentsWe gratefully acknowledge financial support by the Deutsche Forschungsgemeinschaft [(DFG) priority program “Elementary Processes of Organic Photovoltaics”], the German Ministry of Science and Education (BMBF), the Fonds der Chemischen Industrie (Frankfurt, Germany), and BASF SE (Ludwigshafen, Germany). ReferencesG. Yu, J. Gao, J. C. Hummelen, F. Wudl, and A. J. Heeger,
“Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor-acceptor heterojunctions,”
Science, 270 1789
–1791
(1995). http://dx.doi.org/10.1126/science.270.5243.1789 Google Scholar
J. J. M. Halls, C. A. Walsh, N. C. Greenham, E. A. Marseglia, R. H. Friend, S. C. Moratti, and A. B. Holmes,
“Efficient photodiodes from interpenetrating polymer networks,”
Nature, 376 498
–500
(1995). http://dx.doi.org/10.1038/376498a0 Google Scholar
M. Reyes-Reyes, K. Kim, and D. L. Carroll,
“High-efficiency photovoltaic devices based on annealed poly(3-hexylthiophene) and 1-(3-methoxycarbonyl)-propyl-1-phenyl-(6,6)C61 blends,”
Appl. Phys. Lett., 87 083506
(2005). http://dx.doi.org/10.1063/1.2006986 Google Scholar
J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger, and G. C. Bazan,
“Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols,”
Nat. Mater., 6 497
–500
(2007). http://dx.doi.org/10.1038/nmat1928 Google Scholar
Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray, and L. Yu,
“For the bright future—bulk heterojunction polymer solar cells with power conversion efficiency of 7.4,”
Adv. Mater., 22 E135
–E138
(2010). http://dx.doi.org/10.1002/adma.200903528 Google Scholar
M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger, and C. L. Brabec,
“Design rules for donors in bulk-heterojunction solar cells—towards 10% energy-conversion efficiency,”
Adv. Mater., 18 789
–794
(2006). http://dx.doi.org/10.1002/adma.200501717 Google Scholar
P. Schilinsky, U. Asawapirom, U. Scherf, M. Biele, and C. J. Brabec,
“Influence of the molecular weight of poly(3-hexylthiophene) on the performance of bulk heterojunction solar cells,”
Chem. Mater., 17 2175
–2180
(2005). http://dx.doi.org/10.1021/cm047811c Google Scholar
L. Schmidt-Mende, A. Fechtenkötter, K. Müllen, E. Moons, R. H. Friend, and J. D. MacKenzie,
“Self-organized discotic liquid crystals for high-efficiency organic photovoltaics,”
Science, 293 1119
–1122
(2001). http://dx.doi.org/10.1126/science.293.5532.1119 Google Scholar
S. Roquet, A. Cravino, P. Leriche, O. Alévêque, P. Frère, and J. Roncali,
“Triphenylamine−thienylenevinylene hybrid systems with internal charge transfer as donor materials for heterojunction solar cells,”
J. Am. Chem. Soc., 128 3459
–3466
(2006). http://dx.doi.org/10.1021/ja058178e Google Scholar
N. Kopidakis, J. M. William, J. van de Lagemaat, D. S. Ginley, G. Rumbles, S. E. Shaheen, and W. L. Rance,
“Bulk heterojunction organic photovoltaic devices based on phenyl-cored thiophene dendrimers,”
Appl. Phys. Lett., 89 103524
(2006). http://dx.doi.org/10.1063/1.2337859 Google Scholar
C.-Q. Ma, M. Fonrodona, M. C. Schikora, M. M. Wienk, R. A. J. Janssen, and P. Bäuerle,
“Solution-processed bulk-heterojunction solar cells based on monodisperse dendritic oligothiophenes,”
Adv. Funct. Mater., 18 3323
–3331
(2008). http://dx.doi.org/10.1002/adfm.200800584 Google Scholar
W. W. H. Wong, C.-Q. Ma, W. Pisula, C. Yan, X. Feng, D. J. Jones, K. Müllen, R. A. J. Janssen, P. Bäuerle, and A. B. Holmes,
“Self-assembling thiophene dendrimers with a hexa-peri-hexabenzocoronene core−synthesis, characterization and performance in bulk heterojunction solar cells,”
Chem. Mater., 22 457
–466
(2010). http://dx.doi.org/10.1021/cm903272y Google Scholar
L. Valentini, D. Bagnis, A. Marrocchi, M. Seri, A. Taticchi, and J. M. Kenny,
“Novel anthracene-core molecule for the development of efficient PCBM-based solar cells,”
Chem. Mater., 20 32
–34
(2008). http://dx.doi.org/10.1021/cm703011k Google Scholar
W. W. H. Wong, T. B. Singh, D. Vak, W. Pisula, C. Yan, X. Feng, E. L. Williams, K. L. Chan, Q. Mao, D. J. Jones, C.-Q. Ma, K. Müllen, P. Bäuerle, and A. Holmes,
“Solution proceassable fluorenyl hexa-peri-hexabenzocoronenes in organic field-effect transistors and solar cells,”
Adv. Funct. Mater., 20 927
–938
(2010). http://dx.doi.org/10.1002/adfm.200901827 Google Scholar
M. T. Lloyd, A. C. Mayer, S. Subramanian, D. A. Mourey, D. J. Herman, A. V. Bapat, and G. G. Malliaras,
“Efficient solution-processed photovoltaic cells based on an anthradithiophene/fullerene blend,”
J. Am. Chem. Soc., 129 9144
–9149
(2007). http://dx.doi.org/10.1021/ja072147x Google Scholar
F. Silvestri, M. D. Irwin, L. Beverina, A. Facchetti, G. A. Pagani, and T. J. Marks,
“Efficient squaraine-based solution processable bulk-heterojunction solar cells,”
J. Am. Chem. Soc., 130 17640
–17641
(2008). http://dx.doi.org/10.1021/ja8067879 Google Scholar
U. Mayerhöffer, K. Deing, K. Gruß, H. Braunschweig, K. Meerholz, and F. Würthner,
“Outstanding short-circuit currents in BHJ solar cells based on NIR-absorbing acceptor-substituted squaraines,”
Angew. Chem. Int. Ed., 48 8776
–8779
(2009). http://dx.doi.org/10.1002/anie.200903125 Google Scholar
T. Rousseau, A. Cravino, T. Bura, G. Ulrich, R. Ziessel, and J. Roncali,
“Multi-donor molecular bulk heterojunction solar cells: improving conversion efficiency by synergistic dye combinations,”
J. Mater. Chem., 19 2298
–2300
(2009). http://dx.doi.org/10.1039/b903189h Google Scholar
N. M. Kronenberg, M. Deppisch, F. Würthner, H. W. A. Lademann, K. Deing, and K. Meerholz,
“Bulk heterojunction organic solar cells based on merocyanine colorants,”
Chem. Commun., 6489
–6491
(2008). http://dx.doi.org/10.1039/b813341g Google Scholar
A. B. Tamayo, B. Walker, and T.-Q. Nguyen,
“A low band gap, solution processable oligothiophene with a diketopyrrolopyrrole core for use in organic solar cells,”
J. Phys. Chem. C, 112 11545
–11551
(2008). http://dx.doi.org/10.1021/jp8031572 Google Scholar
B. Walker, A. B. Tamayo, X.-D. Dang, P. Zalar, J. H. Seo, A. Garcia, M. Tantiwiwat, and T.-Q. Nguyen,
“Nanoscale phase separation and high photovoltaic efficiency in solution-processed, small-molecule bulk heterojunction solar cells,”
Adv. Funct. Mater., 19 3063
–3069
(2009). http://dx.doi.org/10.1002/adfm.200900832 Google Scholar
F. Würthner, R. Wortmann, and K. Meerholz,
“Chromophore design for photorefractive organic materials,”
ChemPhysChem, 3 17
–31
(2002). http://dx.doi.org/10.1002/1439-7641(20020118)3:1<17::AID-CPHC17>3.0.CO;2-N Google Scholar
A. J. Moulé and K. Meerholz,
“Controlling morphology in polymer-fullerene mixtures,”
Adv. Mat., 20 240
–245
(2008). http://dx.doi.org/10.1002/adma.200701519 Google Scholar
R. J. Kline, M. D. McGehee, E. N. Kadnikova, J. S. Liu, and J. M. J. Fréchet,
“Controlling the field-effect mobility of regioregular polythiophene by changing the molecular weight,”
Adv. Mater., 15 1519
–1522
(2003). http://dx.doi.org/10.1002/adma.200305275 Google Scholar
F. Würthner, S. Yao, T. Debaerdemaeker, and R. Wortmann,
“Dimerization of merocyanine dyes. structural and energetic characterization of dipolar dye aggregates and implications for nonlinear optical materials,”
J. Am. Chem. Soc., 124 9431
–9447
(2002). http://dx.doi.org/10.1021/ja020168f Google Scholar
S. Beckmann, K.-H. Etzbach, P. Krämer, K. Lukaszuk, R. Matschiner, A. J. Schmidt, P. Schuhmacher, R. Sens, G. Seybold, R. Wortmann, and F. Würthner,
“Electrooptical chromophores for nonlinear optical and photorefractive applications,”
Adv. Mater., 11 536
–541
(1999). http://dx.doi.org/10.1002/(SICI)1521-4095(199905)11:7<536::AID-ADMA536>3.0.CO;2-I Google Scholar
H. C. F. Martens, H. B. Brom, and P. W. M. Blom,
“Frequency-dependent electrical response of holes in poly(p-phenylene vinylene),”
Phys. Rev. B, 60 8489
–8492
(1999). http://dx.doi.org/10.1103/PhysRevB.60.R8489 Google Scholar
M. Lenes, F. B. Kooistra, J. C. Hummelen, I. Van Severen, L. Lutsen, D. Vanderzande, T. J. Cleij, and P. W. M. Blom,
“Charge dissociation in polymer:fullerene bulk heterojunction solar cells with enhanced permittivity,”
J. Appl. Phys., 104 114517
(2008). http://dx.doi.org/10.1063/1.3039191 Google Scholar
V. D. Mihailetchi, P. W. M. Blom, J. C. Hummelen, and M. T. Rispens,
“Cathode dependence of the open-circuit voltage of polymer:fullerene bulk heterojunction solar cells,”
J. Appl. Phys., 94 6849
–6854
(2003). http://dx.doi.org/10.1063/1.1620683 Google Scholar
H. B. Michaelson, Handbook of Chemistry and Physics, E
–78 63rd ed.CRC Press, Boca Raton, FL
(1982). Google Scholar
A. Dieckmann, H. Bässler, and P. M. Borsenberger,
“An assessment of the role of dipoles on the density-of-states function of disordered molecular solids,”
J. Chem. Phys., 99 8136
–8141
(1993). http://dx.doi.org/10.1063/1.465640 Google Scholar
A. J. Moulé, J.-B. Bonekamp, and K. Meerholz,
“The effect of active layer thickness and composition on the performance of bulk-heterojunction solar cells,”
J. Appl. Phys., 100 094503
(2006). http://dx.doi.org/10.1063/1.2360780 Google Scholar
|

