|
|
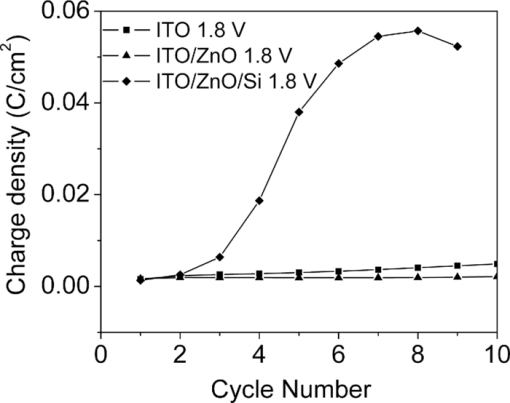
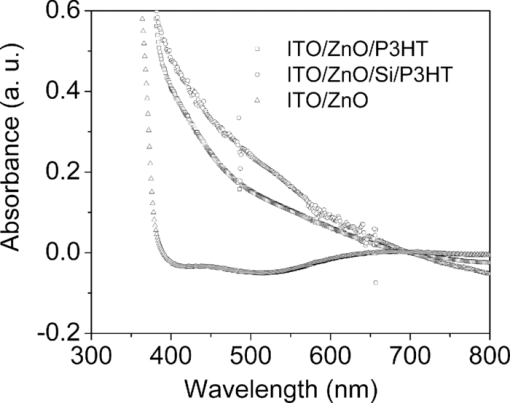
1.IntroductionPolymer hybrid solar cells consisting of conjugated polymers as electron donor and nanostructured inorganic semiconductors as electron acceptor are promising candidates for generating electricity. They have attracted extensive attention1, 2, 3, 4, 5, 6 because they may combine the advantages of both sides: solution processing of polymers and high electron affinity/transport of inorganic semiconductors. In addition to the widely used polymer/fullerene solar cells,7 hybrid organic/inorganic solar cells offer another opportunity to develop a new generation of low-cost solar cells for high performance. The interfacial area between donor and acceptor can also be greatly enhanced by using a variety of solution processable polymers and nanostructured materials.8 Extensive work has already been reported that has demonstrated the promise of combining conjugated polymers with various nanostructured inorganic materials, such as CdSe,1, 9, 10 TiO2,11, 12, 13, 14 ZnO,15, 16 and Si.17 In physically blended polymer-inorganic hybrid solar cells, although charge separation has been improved,8, 18 it is still difficult to control the composite morphology in order to provide an efficient charge transport via inorganic nanocrystals. To overcome this drawback, ordered heterojunction was tried by utilizing one-dimensional (1-D) inorganic semiconductors to realize a continuous charge transport pathway. 1-D inorganic nanostructured templates, including nanorods, nanotubes, and nanowires, vertically aligned on a substrate have a unique design with nanoscale dimensions. In order to achieve an efficient polymer filling and charge transfer from conjugated polymers to inorganic semiconductors, in situ polymerization starting from monomers has been used rather than physically filtrating long chain polymers directly into the nanostructures.19 The monomers have a much smaller size than the nanopores in the inorganic nanostructured template and could significantly improve infiltration. Additionally, chemical modification of the surface of inorganic materials (e.g., TiO2, ZnO, etc.) could create a more favorable surface energy.20 Phosphonic acid21 and amine moieties22 have been used as an interfacial molecular layer between the donor and acceptor. Zhang reported that a chemical oxidative polymerization has been applied to in situ polymerize P3HT inside a surface-initiated TiO2 nanotube template, and their results showed improved photophysical properties compared to the physically filtrated samples.19 Tepavcevic used UV irradiation to grow P3HT and found that the photocurrent response was more enhanced than that in the samples made by physical filtration.20 In this study, P3HT was in situ polymerized onto a ZnO nanowire acceptor template by electrochemical polymerization through a thienylsilane linker. The ZnO nanowires were grown perpendicularly onto an ITO substrate from aqueous solution at low temperature. The electrochemical polymerization of P3HT was conducted on clean ITO substrate, ZnO nanowire surface with and without a silane linker, respectively. The silane linker was capped with a thiophene ring to improve the interaction between P3HT and ZnO nanowires. The results show that the thienylsilane modified layer obviously decreased the oxidation potential for the electrochemical polymerization of P3HT. 2.Experiment MethodsAnhydrous toluene and acetonitrile were purchased from Acros (Morris Plains, NJ, USA). Triethoxy-2-thienylsilane and tetrabutylammonium hexafluorophonate (Bu4NFP6, electrochemical grade) were obtained from Sigma Aldrich (St. Louis, MO, USA). They were used as received. Chloroform was dried by CaH2. All the other solvents were ordered from commercial sources and used without further purification. 3-hexylthiophene was synthesized according to a literature method.23 ZnO nanowires were prepared by a method reported previously.24 They were vertically aligned on an ITO substrate with a hexagonal shape of an average diameter of ∼50 nm. ZnO nanoparticle (NP) films were prepared by spin-coating the solution of 0.75 M zinc acetate dehydrate, 0.75 M ethanol amine, and 2-methoxyethanol solution for multiple times, followed by annealing the films at 350°C for 2 h. Scanning electron microscopy (SEM) was performed via Hitachi S-4300N SEM at an accelerating voltage of 10 kV at room temperature. Water contact angle images were taken from a Video Contact Angle 2000 system via sessile drop method. O2 plasma cleaning was done using PDC-32G plasma cleaner (Harrick Plasma). Cyclic voltammetry (CV) experiments were conducted with an Ametek versa SATA 3 instrument. X-ray photoelectron spectroscopy (XPS) measurements were performed on an SSX-100 system (Surface Science Instruments) equipped with a monochromated Al Kα x-ray source, a hemispherical sector analyzer, and a resistive anode detector. UV-visible (UV-Vis) absorption spectra were obtained on an HP Agilent 8352 UV-Vis spectrophotometer. 2.1.Surface-Modification of ZnO NanowiresThe surface modification of ZnO nanowires was conducted following a reported method.25 The ZnO nanowire template was placed into an O2 plasma cleaner for 15 min to promote hydroxylation with the ZnO side facing up. A silane solution was freshly prepared by mixing triethoxy-2-thienylsilane molecules with toluene and n-butylamine catalyst. The solution was preheated at 45°C for 30 min, and then the pretreated ITO/ZnO nanowire substrate was immersed into the heated solution. After that, the ITO/ZnO nanowire substrate was removed from the solution and rinsed with toluene and acetone, and dried with N2. Postcuring was done at 110°C. 2.2.Electrochemical PolymerizationAn enclosing homemade glass cell was installed using a standard three-electrode configuration with an Ag/AgCl as the reference electrode and a coiled platinum auxiliary electrode as the counter electrode. Different substrates, including ITO and ITO/ZnO nanowires with and without surface modification, served as the working electrode, respectively. CV experiments were conducted at room temperature. The electrolyte consisting of Bu4NFP6 (0.1 M) and 3-hexylthiophene (3HT) monomer (0.005 M) in an anhydrous acetonitrile solution was freshly prepared. The maximum applied potentials for electrochemical polymerization were varied from 1.5 to 2.2 V with a scan rate of 0.1 V/s for each. The final films grown on the ZnO surface were washed with ethanol, acetone and then dried by N2. 3.Results and Discussion3.1.Surface Modification of ZnO NanowiresFigure 1 shows the SEM images of the ZnO nanowires. It can be seen that the prepared ZnO nanowires have an average diameter of ∼50 nm and a length of ∼1 μm. They are uniformly distributed on the ITO substrate. Fig. 1(a) Top view and (b) tilted cross-sectional SEM image of the ZnO nanowires grown on an ITO substrate. 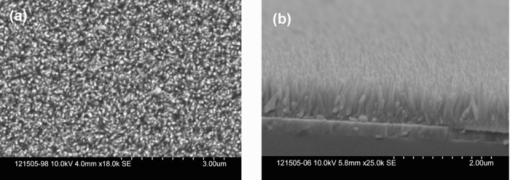 The electrochemical polymerization method is an easy and cheap method to obtain polymers. In addition, it does not require polymers to have a side chain for solubility. In order to grow polymers by the electrochemical method, the conductive substrates are required; however this can be a further advantage for solar cell application because solar cells are fabricated using conducting substrate as electrode, such as ITO, FTO-coated glass, or plastic substrates. There has been extensive work in the electrochemical polymerization field; polythiophene,26, 27, 28 polypyrrole,29 and poly(3,4-ethylenedioxythiophene)30 have been electrochemically polymerized and well studied. One disadvantage for electrochemical polymerization method on conducting substrate is that it initiates the polymerization reaction at the electrode surface rather than that of the semiconducting oxide (e.g., ZnO),20 and therefore, it may lead to a large amount of polymer formed on the substrate (electrode) rather than directly onto the semiconducting oxide. To solve this issue, the ZnO nanowires were surface modified by a layer of thienylsilane molecules capped with a thiophene ring that may promote in situ polymerization of P3HT on the ZnO nanowire surface. It would possibly help to form a homogeneous layer of P3HT directly attached onto the ZnO nanowire surface. Figure 2 (a → b) shows the process of surface functionalization of ZnO nanowires using a thienylsilane molecule, followed by the formation of P3HT synthesized by in situ electrochemical polymerization (b → c). Fig. 2Procedure to functionalize the ZnO nanowires using triethoxy-2-thienylsilane and then electrochemically polymerize P3HT directly onto the ZnO nanowire surface. 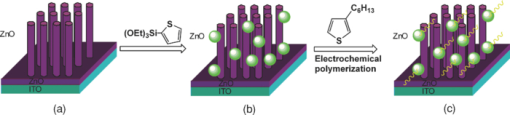 After the surface functionalization, the ZnO nanowire templates were characterized by XPS measurement as shown in Fig. 3. The chemical nature and surface energy of ZnO nanowires are expected to change after the modification. Figure 3 is the wide-range survey spectrum. The C (1s) signals containing three components at 285.000, 286.666, and 288.921 eV were fitted by Gaussian–Lorentzian functions. From the XPS spectra, the Si (2p) emission around 103.0 eV was found to be weak, but it could still be identified in Fig. 3. This confirms that the silane molecular layer formed on ZnO surface. Fig. 3(a) XPS survey spectrum of thienylsilane modified ZnO nanowires and (b) zoomed XPS in the binding energy of 100–200 eV region that shows the Si (2p) signal. 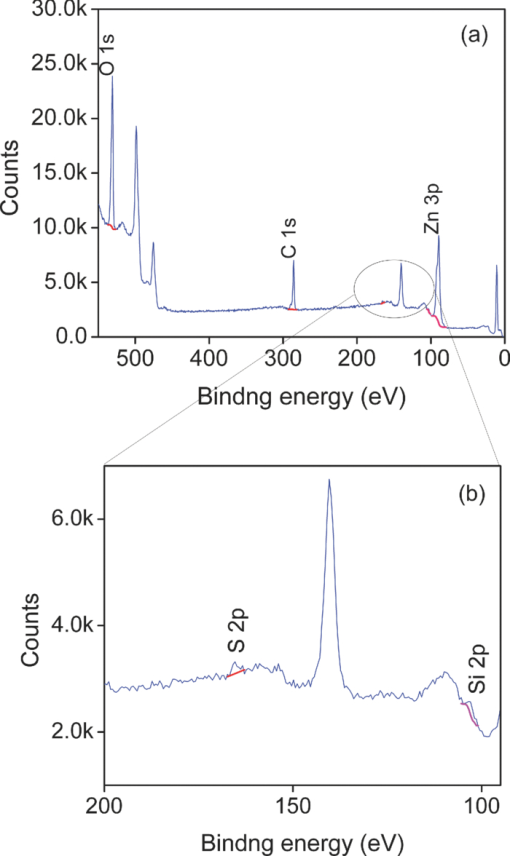 To further identify the surface modification on ZnO nanowires, water contact angle (WCA) measurements were conducted to monitor the surface properties of ZnO samples before and after surface modification. Because the surface properties of nanowires are difficult to be studied directly by this method, the ZnO NP films were prepared and measured instead. This method can be a good indicator to monitor the properties of surface-modified ZnO films. At least two samples were tested, and no less than five spots on each sample were examined to ensure accurate measurements. Figure 4 shows the WCA results of the surface-modification processes. The WCA of the pristine ZnO NP film was around 42 ± 2 deg [Fig. 4] and it became <20 deg [Fig. 4] after the O2 plasma treatment, which caused a hydrophilic ZnO surface. This result is consistent with the O2 plasma-treated ZnO film reported by others.25, 31, 32 Such hydroxylated treatment can promote the reactivity of ZnO sample with the silane reagent. After the silanization reaction, a WCA of 75 ± 2 deg [Fig. 4] was observed from multiple spots on different slides. This data are quite similar to the phenyltriethoxysilane C6H5Si(OCH2CH3)3 (PTES)-modified ZnO nanoparticle surface, which has a WCA of 72.5 ± 4.3 deg.25 This confirmed that the silane molecules attached onto the surface of ZnO films successfully and changed the ZnO surface properties. Because of the hydrophobic nature of the thiophene ring, the contact angle of ZnO sample after silanization increased greatly. In addition, the contact angle of silanized samples kept consistent after several days, which showed this surface modification is quite stable. 3.2.Electropolymerization of P3HT film on ITO/ZnO nanowire substrateIn order to study the effect of thienylsilane functionalization treatment on the electrochemical deposition of P3HT, the maximum applied potentials (Vmax) in the potentiodynamic CV experiments were varied quantitatively on different substrates: (i) ITO substrate, (ii) ITO/ZnO nanowires without thienylsilane group surface modification, and (iii) ITO/ZnO nanowires with thienylsilane group surface modification. In the rest of the work, they will be labeled as ITO, ITO/ZnO, ITO/ZnO/Si, respectively. P3HT was deposited by sweeping the working electrode potential from 0 to Vmax (verse Ag/Ag+) at a scan rate of 0.1 V/s for a specific number of cycles. The representative CV sweeps of growing P3HT on three different substrates (ITO, ITO/ZnO, ITO/ZnO/Si) at a Vmax of 1.8 and 1.9 V, respectively, are shown in Fig. 5. The evidence for successful P3HT growth was the increase of the anodic and cathodic peak current in each successive cycle. During the experiments, a dark color film was observed to form on the electrodes. By increasing Vmax, the area beneath each curve was increased, which indicated that more P3HT was formed on the electrode. Fig. 5Cyclic voltammogram of the 3-hexylthiophene monomer at different substrate ITO (a) 1.8 V, (d) 1.9 V; ITO/ZnO (b) 1.8 V, (e) 1.9 V, and ITO/ZnO/Si (c) 1.8 V, (f) 1.9 V. 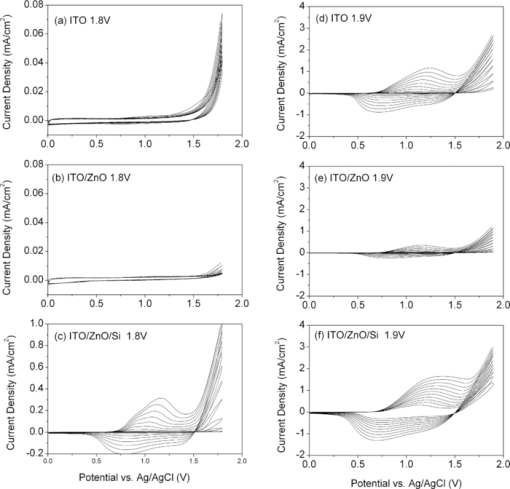 As shown in Fig. 5, when the number of cycles increased, the oxidization and reduction were also subsequently changed. When comparing the polymerization under the same Vmax, the area inside the CV curves was in the order of ITO/ZnO < ITO < ITO/ZnO/Si for both Vmax = 1.8 V and Vmax = 1.9 V. The reason for ITO/ZnO < ITO was that ITO had a conducting surface, but ZnO had a semiconducting surface; therefore, P3HT was easier to be oxidized on the ITO substrate than that of ITO/ZnO substrate. However, compared to the ZnO nanowire substrate with thienylsilane surface modification, the P3HT polymerization onset potential was greatly decreased. This indicated that the thienylsilane functionalized ZnO surface acted as a favorable nucleation site for electrochemical polymerization and led to a low-surface electrical potential for nucleation. For a more quantitative analysis and comparison of the effects of substrates and variations in Vmax on P3HT growth, the charge density for each curve was calculated. The charge density versus numbers of CV sweeps obtained from the anodic waves were plotted in Fig. 6. It was clearly found that the charge density for ITO and ITO/ZnO substrate changed insignificantly at the Vmax of 1.8 V. However, it was obviously increased for the ITO/ZnO/Si substrate after the self-assembling of a molecular layer of thienylsilane linker. This further proved that the surface modification could enhance P3HT polymerization at lower potentials. 3.3.Optical Absorption of Electropolymerized P3HT FilmFigure 7 shows the UV-visible absorption spectra of the ZnO nanowires and electropolymerized P3HT films with and without thienylsilane surface modification. The ZnO nanowires show absorption up to 375 nm with a characteristic shape. In the presence of P3HT, the absorption was broadened to 650 nm for both the samples of ZnO nanowires with and without surface modification. In addition, it was evident that the UV-Vis absorption was much stronger in the sample with the thienylsilane surface modification than the sample without it. The reason is that the thienylsilane-functionalized layer acted as a favorable nucleation site for electrochemical polymerization and caused more P3HT to grow onto ZnO nanowires. This is consistent with the higher charge density found in the sample with thienylsilane surface modification. 4.ConclusionsP3HT was electropolymerized onto the vertically aligned thienylsilane-modified ZnO nanowires. The polymerization onset potential decreased for the ZnO nanowires with thienylsilane group surface modification compared to that of the pristine ZnO nanowires. By increasing the maximum applied potential (Vmax) for polymerization, an increase in the area beneath each curve indicated that more P3HT was formed. The silane-based surface functionalization acted as a favorable nucleation site for electrochemical polymerization. The UV-Vis absorption in the ZnO nanowires with thienylsilane surface modification was much stronger than that for ZnO nanowires without surface modification. AcknowledgmentsThis project was partially supported by the National Science Foundation (Grant No. ECCS-0950731), NASA EPSCoR (Grant No. NNX09AP67A), ACS Petroleum Research Funds DNI (Grant No. 48733DNI10), US-Israel Binational Science Foundation (Grant No. 2008265), and U.S.-Egypt Joint Science & Technology Funds (Grant No. 913), the MRSEC Program of the National Science Foundation under Award No. DMR-0819885. The authors also thank Dr. Bing Luo, College of Science and Engineering Characterization Facility, University of Minnesota, for conducting XPS measurement, part of the NSF-funded Materials Research Facilities Network ( www.mrfn.org). ReferencesW. U. Huynh, J. J. Dittmer, and A. P. Alivisatos,
“Hybrid nanorod-polymer solar cells,”
Science, 295 2425
–2427
(2002). http://dx.doi.org/10.1126/science.1069156 Google Scholar
T. Xu, and Q. Qiao,
“Conjugated polymer-inorganic semiconductor hybrid solar cells,”
Energy Environ. Sci.,
(2011). http://dx.doi.org/10.1039/C0EE00632G Google Scholar
H. Borchert,
“Elementary processes and limiting factors in hybrid polymer/nanoparticle solar cells,”
Energy Environ. Sci., 3 1682
–1694
(2010). http://dx.doi.org/10.1039/c0ee00181c Google Scholar
I. Gonzalez-Valls, and M. Lira-Cantu,
“Vertically-aligned nanostructures of ZnO for excitonic solar cells: A review,”
Energy Environ. Sci., 2 19
–34
(2009). http://dx.doi.org/10.1039/b811536b Google Scholar
M. Memesa, S. Weber, S. Lenz, J. Perlich, R. Berger, P. Muller-Buschbaum, and J. S. Gutmann,
“Integrated blocking layers for hybrid organic solar cells,”
Energy Environ. Sci., 2 783
–790
(2009). http://dx.doi.org/10.1039/b902754h Google Scholar
Y. F. Zhou, M. Eck, and M. Kruger,
“Bulk-heterojunction hybrid solar cells based on colloidal nanocrystals and conjugated polymers,”
Energy Environ. Sci., 3 1851
–1864
(2010). http://dx.doi.org/10.1039/c0ee00143k Google Scholar
M. Siddiki, J. Li, D. Galipeau, and Q. Qiao,
“A review on polymer multijunction solar cells,”
Energy Environ. Sci., 3 867
–883
(2010). http://dx.doi.org/10.1039/b926255p Google Scholar
J. Bouclé, P. Ravirajan, and J. Nelson,
“Hybrid polymer–metal oxide thin films for photovoltaic applications,”
J. Mater. Chem., 17 3141
–3153
(2007). http://dx.doi.org/10.1039/b706547g Google Scholar
S. Dayal, N. Kopidakis, D. C. Olson, D. S. Ginley, and G. Rumbles,
“Photovoltaic devices with a low band gap polymer and CdSe nanostructures exceeding 3% efficiency,”
Nano Lett., 10 239
–242
(2010). http://dx.doi.org/10.1021/nl903406s Google Scholar
W. U. Huynh, X. Peng, and A. P. Alivisatos,
“CdSe nanocrystal rods/poly(3-hexylthiophene) composite photovoltaic devices,”
Adv. Mater., 11 923
–927
(1999). http://dx.doi.org/10.1002/(SICI)1521-4095(199908)11:11<923::AID-ADMA923>3.0.CO;2-T Google Scholar
Q. Qiao, and J. T. McLeskey,
“Water-soluble polythiophene/nanocrystalline TiO2 solar cells,”
Appl. Phys. Lett., 86 153501
(2005). http://dx.doi.org/10.1063/1.1900300 Google Scholar
Q. Qiao, Y. Xie, and J. J. T. McLeskey,
“Organic/inorganic polymer solar cells using a buffer layer from all-water-solution processing,”
J. Phys. Chem. C, 112 9912
–9916
(2008). http://dx.doi.org/10.1021/jp7115615 Google Scholar
Y.-Y. Lin, T.-H. Chu, S.-S. Li, C.-H. Chuang, C.-H. Chang, W.-F. Su, C.-P. Chang, M.-W. Chu, and C.-W. Chen,
“Interfacial nanostructuring on the performance of polymer/TiO2 nanorod bulk heterojunction solar cells,”
J. Am. Chem. Soc., 131 3644
–3649
(2009). http://dx.doi.org/10.1021/ja8079143 Google Scholar
Q. Qiao, L. Su, J. Beck, and J. T. McLeskey,
“Characteristics of water soluble polythiophene:TiO2 composite and its application in photovoltaics,”
J. Appl. Phys., 98 094906
(2005). http://dx.doi.org/10.1063/1.2130517 Google Scholar
W. J. E. Beek, M. M. Wienk, and R. A. J. Janssen,
“Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer,”
Adv. Mater., 16 1009
–1013
(2004). http://dx.doi.org/10.1002/adma.200306659 Google Scholar
D. J. D. Moet, L. J. A. Koster, B. de Boer, and P. W. M. Blom,
“Hybrid polymer solar cells from highly reactive diethylzinc: MDMO–PPV versus P3HT,”
Chem. Mater., 19 5856
–5861
(2007). http://dx.doi.org/10.1021/cm070555u Google Scholar
S.-C. Shiu, J.-J. Chao, S.-C. Hung, C.-L. Yeh, and C.-F. Lin,
“Morphology dependence of silicon nanowire/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) heterojunction solar cells,”
Chem. Mater., 22 3108
–3113
(2010). http://dx.doi.org/10.1021/cm100086x Google Scholar
J. Bouclé, S. Chyla, M. S. P. Shaffer, J. R. Durrant, D. D. C. Bradley, and J. Nelson,
“Hybrid solar cells from a blend of poly(3-hexylthiophene) and ligand-capped TiO2 nanorods,”
Adv. Funct. Mater., 18 622
–633
(2008). http://dx.doi.org/10.1002/adfm.200700280 Google Scholar
Y. Zhang, C. Wang, L. Rothberg, and M.-K. Ng,
“Surface-initiated growth of conjugated polymers for functionalization of electronically active nanoporous networks: Synthesis, structure and optical properties,”
J. Mater. Chem., 16 3721
–3725
(2006). http://dx.doi.org/10.1039/b605421h Google Scholar
S. Tepavcevic, S. B. Darling, N. M. Dimitrijevic, T. Rajh, and S. J. Sibener,
“Improved hybrid solar cells via in situ UV polymerization,”
Small, 5 1776
–1783
(2009). http://dx.doi.org/10.1002/smll.200900093 Google Scholar
C.-W. Hsu, L. Wang, and W.-F. Su,
“Effect of chemical structure of interface modifier of TiO2 on photovoltaic properties of poly(3-hexylthiophene)/TiO2 layered solar cells,”
J. Colloid Interface Sci., 329 182
–187
(2009). http://dx.doi.org/10.1016/j.jcis.2008.10.008 Google Scholar
W. J. E. Beek, M. M. Wienk, M. Kemerink, X. Yang, and R. A. J. Janssen,
“Hybrid zinc oxide conjugated polymer bulk heterojunction solar cells,”
J. Phys. Chem. B, 109 9505
–9516
(2005). http://dx.doi.org/10.1021/jp050745x Google Scholar
D. Appelhans, D. Ferse, H. J. P. Adler, W. Plieth, A. Fikus, K. Grundke, F. J. Schmitt, T. Bayer, and B. Adolphi,
“Self-assembled monolayers prepared from ω-thiophene-functionalized n-alkyltrichlorosilane on silicon substrates,”
Colloids Surf., A, 161 203
–212
(2000). http://dx.doi.org/10.1016/S0927-7757(99)00338-6 Google Scholar
J.-S. Huang, and C.-F. Lin,
“Influences of ZnO sol-gel thin film characteristics on ZnO nanowire arrays prepared at low temperature using all solution-based processing,”
J. Appl. Phys., 103 014304
(2008). http://dx.doi.org/10.1063/1.2828172 Google Scholar
C. G. Allen, D. J. Baker, J. M. Albin, H. E. Oertli, D. T. Gillaspie, D. C. Olson, T. E. Furtak, and R. T. Collins,
“Surface modification of ZnO using triethoxysilane-based molecules,”
Langmuir, 24 13393
–13398
(2008). http://dx.doi.org/10.1021/la802621n Google Scholar
J. T. Sullivan, K. E. Harrison, J. P. Mizzell, and S. M. Kilbey,
“Contact angle and electrochemical characterization of multicomponent thiophene-capped monolayers,”
Langmuir, 16 9797
–9803
(2000). http://dx.doi.org/10.1021/la000225n Google Scholar
J. P. Correia, and L. M. Abrantes,
“In situ ellipsometric studies on the electrochemically induced structural modifications during poly(3-methylthiophene) formation,”
Synth. Met., 156 287
–292
(2006). http://dx.doi.org/10.1016/j.synthmet.2005.12.013 Google Scholar
M. Vignali, R. A. H. Edwards, M. Serantoni, and V. J. Cunnane,
“Electropolymerized polythiophene layer extracted from the interface between two immiscible electrolyte solutions: Current-time analysis,”
J. Electroanal. Chem., 591 59
–68
(2006). http://dx.doi.org/10.1016/j.jelechem.2006.03.033 Google Scholar
C. M. Li, C. Q. Sun, W. Chen, and L. Pan,
“Electrochemical thin film deposition of polypyrrole on different substrates,”
Surf. Coat. Technol., 198 474
–477
(2005). http://dx.doi.org/10.1016/j.surfcoat.2004.10.065 Google Scholar
A. I. Melato, A. S. Viana, and L. M. Abrantes,
“Different steps in the electrosynthesis of poly(3,4-ethylenedioxythiophene) on platinum,”
Electrochim. Acta, 54 590
–597
(2008). http://dx.doi.org/10.1016/j.electacta.2008.07.030 Google Scholar
M. D. K. Ingall, C. H. Honeyman, J. V. Mercure, P. A. Bianconi, and R. R. Kunz,
“Surface functionalization and imaging using monolayers and surface-grafted polymer layers,”
J. Am. Chem. Soc., 121 3607
–3613
(1999). http://dx.doi.org/10.1021/ja9833927 Google Scholar
B. Zhang, T. Kong, W. Xu, R. Su, Y. Gao, and G. Cheng,
“Surface functionalization of zinc oxide by carboxyalkylphosphonic acid self-assembled monolayers,”
Langmuir, 26 4514
–4522
(2010). http://dx.doi.org/10.1021/la9042827 Google Scholar
|