|
|
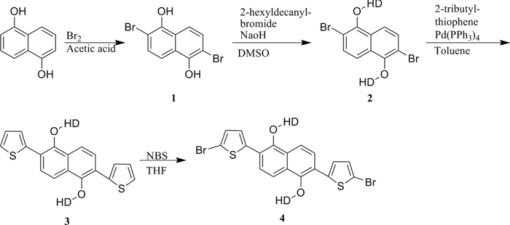
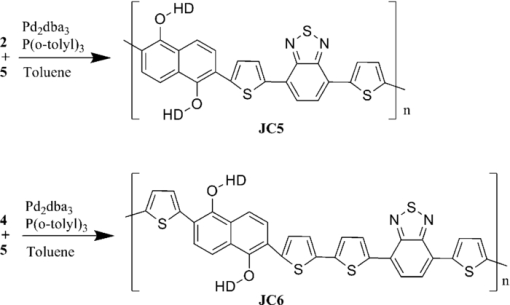
1.IntroductionThe polymer-based solar cells have attracted a lot of interest due to the potential of high volume production at low price.1, 2, 3, 4, 5, 6, 7, 8 A lot of the work has been aimed toward obtaining high power conversion efficiency for small laboratory devices.9, 10, 11 Just as important in the polymer solar cell research but much less explored is degradation/stability8 and big scale processing issues.12, 13, 14 Throughout the recent years a major effort in polymer photovoltaic researches is the synthesis of so-called low bandgap polymers with a bandgap below 2.0 eV. The low bandgap can secure a larger harvest of the photons due to a better match with the sun spectrum, whereby a higher current can be achieved. An approach to prepare low bandgap polymers is by introducing alternating donor and acceptor groups in the conjugated polymer system. This strategy has been proven successful as shown by different groups, lately by University of Chicago and Solarmer Energy, Inc. who has reported devices with efficiencies over 7% and internal quantum efficiency near 100%.15 This is well above the limit reached for the more established and researched P3HT polymer.16 Several different types of low bandgap polymers which consist of donor and acceptor units have been synthesized. The incorporation of alternating donor and acceptor gives rise to charge transfer transition in addition to the π → π* transition. The charge transfer takes place from the highest occupied molecular orbital of the donor group to the lowest unoccupied molecular orbital of the acceptor group. Both the donor and acceptor groups can be varied and a great number of polymers are therefore possible. Mostly used are thiophene-derivatives as donor and benzothiadiazole (BT)-derivatives as the acceptor.6, 17, 18 By designing donor and acceptor groups it is possible to adjust the absorption spectrum, the bandgap, and define specific photovoltaic properties. An important outcome is a possible better coverage of the solar spectrum that can increase the short circuit current density (Jsc) through an increase in the number of absorbed photons, resulting in a higher efficiency (ηe). The lower bandgap can also work in the other way by decreasing the open circuit voltage (Voc). Many other factors, such as the degree of conjugation, the torsion angle between the units in the backbone, as well as the packing of the polymer molecules in the solid phase and many others, influence how a polymer can perform in a photovoltaic device. The optimal polymer, for use in photovoltaic devices, does at the moment, not seem open to rational design and at present this is resolved through a trial and error process. Here we present six different polymers, see Fig. 1, based on thiophene substituted dialkoxybenzene or dialkoxynaphthalene as donor and benzothiadiazole as acceptor. The use of either dialkoxybenzene or dialkoxynaphthalene allows for a simple way to introduce two alkoxy groups to ensure good solubility and processability of the polymer, when applied in photovoltaic devices. By exchanging the benzene with naphthalene, the degree of backbone planarity should be increased, thereby reducing the bandgap of the polymer.17 The synthesis of the polymers JC1 to JC4, their optical properties, and their application in photovoltaic devices has been reported earlier by Carlé19 In this paper two new polymers are presented: JC5 and JC6. The optical properties of these polymers, together with their application in photovoltaic devices, are presented and compared with the properties of polymers JC1 to JC4. Fig. 1The six different polymers tested for optical and photovoltaic properties, EH = 2-ethylhexyl, HD = 2-hexyldecanyl. 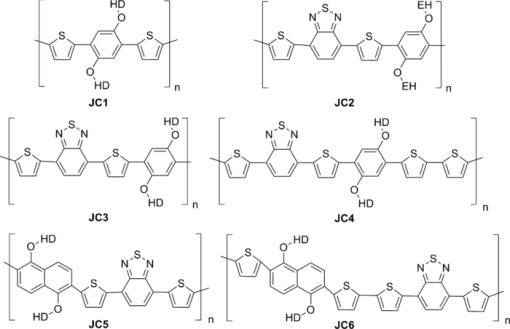 The polymer JC1 is purely a donor-type because of the alternating electron-rich dialkoxybenzene and bithiophene units, in ways similar to, e.g., P3HT. JC2 and JC3 have, besides the donor groups, dialkoxybenzene and thiophene, and also contains the acceptor group BT connected via thiophene groups. JC2 and JC3 have different alkyl side chains being either 2-ethylhexyl or 2-hexyldecyl. JC4 has, instead of single thiophene as JC3, bithiophene groups. The polymers JC5 and JC6 can be compared with the JC3 and JC4 polymers, respectively, as the only difference between these is the substitution of benzene for naphthalene. This series of polymers makes it possible to investigate the consequence of introducing benzothiadiazole units (JC1 → JC2), varying the length of the alkyl side chains (JC2 → JC3), varying the number of thiophene in the backbone (JC3 → JC4) (JC5→JC6) and at last, changing benzene with naphthalene (JC3 → JC5) (JC4 → JC6). 2.Experimental Details1.1.SynthesisThe synthetic procedure of JC1, JC2, JC3, and JC4 can be found in Ref. 19. 2.1.1.2,6-dibromonaphthalene-1,5-diol (1)Naphthalene-1,5-diol (10 g, 62.4 mmol) was issolved in acetic acid (350 ml). Few crystals of iodine were added and the solution heated to 80°C. Bromine (19.95 g, 125 mmol) was dissolved in acetic acid (35 ml) and added over half an hour. The solution was stirred at 80°C for an hour and then cooled to room temperature. Water was added and the precipitate was filtered off, washed with petrol ether, and recrystallized from acetic acid to give the product. Yield: 16.32 g, 82%. 1H NMR (CDCl3, 250 MHz) δ: 7.03 (s, 2H), 9.79 (s, 2H). 13C NMR (CDCl3, 500 MHz) δ: 106.4, 115.8, 126.8, 130.2, 150.0. 2.1.2.2,6-dibromo-1,5-bis(2-hexyldecyloxy)naphthalene (2)1 (4 g, 12.58 mmol) and NaOH (5.03 g, 126 mmol) were dissolved in degassed dimethyl sulfoxide (DMSO) (25 ml). 2-hexyldecanyl bromide (7.68 g, 25.2 mmol) was dissolved in degassed DMSO (25 ml) and added drop wise to the solution. The mixture was heated to 80°C where it was stirred overnight. The mixture was poured into ice water and extracted with dichloromethane (DCM). The phases were separated and the organic phase was washed with water twice, dried over MgSO4, and filtered. The solvent was evaporated under reduced pressure and purified by column chromatography on silica gel using heptane/EtOAc (1:4) as eluent. Yield: 6.8 g, 70%. 1H NMR (CDCl3, 250 MHz) δ: 0.82 (m, 6H), 1.17 to 1.57 (m, 48H), 1.9 (m, 2H), 3.93 (d, 4H), 7.58 (d, 2H), 7.71 (d, 2H). 13C NMR (CDCl3, 500 MHz) δ: 14.4, 22.7, 26.9, 27.0, 29.4, 29.6, 29.7, 30.1, 31.2, 31.7, 31.9, 39.3, 113.7, 119.2, 130.1, 131.1, 152.9. 2.1.3.2,2 ′-(1,5-bis(2-hexyldecyloxy)naphthalene-2,6-diyl)dithiophene (3)2 (1.5 g, 1.96 mmol) and 2-(tributyltin)-thiophene (1.83 g, 4.89 mmol) was dissolved in dry toluene and the catalyst tetrakis triphenylphosphine palladium(0) (500 mg) was added and the solution was stirred at reflux overnight. The solvent was evaporated under vacuum and the product purified by column chromatography on silica gel using heptane/EtOAc (1:9) as eluent. Yield: 1.4 g, 93%. 1H NMR (CDCl3, 500 MHz) δ: 0.89 (m, 6H), 1.26 (m, 48H), 1.41 (m, 4H), 1.55 (m, 4H), 1.90 (m, 2H), 3.74 (d, 4H), 7.15 (q, 2H), 7.41 (dd, 2H), 7.58 (dd, 2H), 7.74 (d, 2H), 7.95 (d, 2H). 13C NMR (CDCl3, 500 MHz) δ: 14.1, 14.12, 22.6, 26.78, 26.81, 29.3, 29.6, 29.7, 30.1, 31.2, 31.90, 31.92, 39.1, 118.8, 123.6, 126.1, 127.0, 127.2, 129.6, 139.5, 151.9. 2.1.4.5,5 ′-(1,5-bis(2-hexyldecyloxy)naphthalene-2,6-diyl)bis(2-bromothiophene)(4)3 (500 mg, 0.65 mmol) was dissolved in THF (15 ml). A solution of N-bromosuccinimide (230 mg, 1.3 mmol) in tetrahydrofuran (THF) was added to the solution in small portions in the dark and then stirred under argon at room temperature for 2 h. Water and diethyl ether were added and the phases separated. The organic phase was washed with water and dried over MgSO4, filtered, and the solvent evaporated under reduced pressure. It purified by column chromatography on silica gel using heptane/EtOAc (1:50) as eluent. Yield: 562 mg, 93%. 1H NMR (CDCl3, 500 MHz) δ: 0.89 (m, 6H), 1.26 (m, 48H), 1.41 (m, 4H), 1.55 (m, 4H), 1.90 (m, 2H), 3.74 (d, 4H), 7.10 (d, 2H), 7.31 (d, 2H), 7.68 (d ,2H), 7.91 (d, 2H). 2.1.5.General procedure for Stille cross-coupling polymerizationOne equivalent of the acceptor monomer 5 and the appropriate donor monomer 2 or 4 were dissolved in degassed toluene. Trio-o-tolylphosphine (4 mol%) and tris-(dibenzylidene acetone) dipalladium(0) (0.5 mol%) were added and the solution was brought to reflux where it was stirred for at least 24 h. The solvent was evaporated under vacuum and the product was dissolved in a minimum amount of boiling chloroform. The polymer was precipitated in about 10 times the volume methanol. The suspension was filtered to give the polymer which was purified by Soxhlet extraction, first with methanol, then hexane, and finally chloroform. The chloroform fraction was evaporated under vacuum to a minimum volume, still keeping the polymer in solution. The polymer was then precipitated by pouring it into 10 times the volume methanol. The suspension was filtered and dried in a vacuum oven to give the purified polymer.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 2.2.Device PreparationThe prefabricated glass substrates coated with a patterned indium tin oxide (ITO) with an active area of 0.5 cm2 was first ultrasonically cleaned in water and then 2-propanol. Poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) (PEDOT:PSS) was spin-coated on top, the electrodes cleaned with water, and then dried at 150°C. The substrates were transferred to a glove box where they were heated at 150°C for 5 min. The active layer of polymer and [6,6]-phenyl-C61-butyric acid methyl ester ([60]PCBM) in dichlorobenzene, with a concentration of 50 mg/ml, were spin-coated on the PEDOT:PSS layer and allowed to dry, and the contacts cleaned with dichlorobenzene. They were transferred to a vacuum chamber, where the aluminum electrode was applied by thermal evaporation at a pressure below 6 × 10−6 mBar. The system was brought to atmospheric pressure and the solar cells were analyzed immediately after. 3.Results and Discussion3.1.SynthesisThe synthesis of the two donor monomers 2,6-dibromo-1,5-bis(2-hexyldecyloxy)naphthalene (2) and 5,5’-(1,5-bis(2-hexyldecyloxy)naphthalene-2,6-diyl)bis(2-bromothiophene) (4) is outlined in Fig. 2. Here the naphthalene is first bromated in acetic acid using bromine and then alkylated with 2-hexyldecyl bromide in the presence of sodium hydroxide in DMSO. Introduction of thiophene groups is done by using 2-tributyltin-thiophene in a Stille coupling with Pd(0) catalyst in dry toluene. The final monomer was obtained by bromation with N-bromosuccinimide. The final donor monomers, 2 and 4, were coupled with the acceptor monomer 4,7-bis(5-(tributylstannyl)thiophen-2-yl)benzo[c][1,2,5]thiadiazole (5), seen in Fig. 3, through a Stille cross coupling polymerization to give the polymers JC5 and JC6 as seen in Fig. 4. Fig. 3The acceptor monomer benzothiadiazole coupled with thiophene. Shown here with tributyltin, which makes it applicable in a Stille cross-coupling polymerization. For synthetic procedure of 5 see Ref. 19. 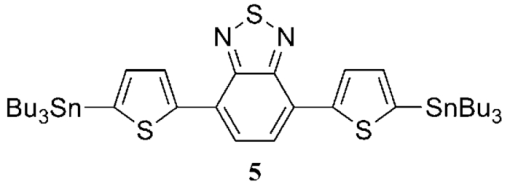 3.2.Characterization of the PolymersThe polymers were characterized by size exclusion chromatography and showed molecular weights of 4.000 Mw (JC6) to 15.000 Mw (JC5), see Table 1. This is relative to low molecular weights, which could be due to low solubility. Table 1Molecular weight and optical data for the six polymers.
Absorption spectra of each polymer were acquired from 320 to 800 nm in both chloroform solution and as thin films spin-coated on glass substrates from a chloroform solution, see Fig. 5. From the spectra it can be seen that JC1 is the only polymer that shows single peak absorption (476 nm), the π → π* transition, and it also has the highest bandgap, 2.2 eV. This is expected, because JC1 does not have incorporated BT acceptor units. The five other polymers show, besides the absorption in the area of 349 to 439 nm, another strong absorption band in the area of 530 to 570 nm, in solution. This band is presumably due to a charge transfer (CT) transition between the thiophene, benzene, or naphthalene donor and the BT acceptor unit similar to what has been observed for other polymers consisting of alternating donor and acceptor units. The absorption spectra are redshifted for the films compared to the solutions for all polymers except for JC1. The λmax of the CT transition of the polymers JC2, JC3, and JC4 are almost the same for the films, 607 to 613 nm, whereas the naphthalene contain polymers JC5 and JC6, and has lower and not similar λmax, 552 and 580 nm, respectively. In the naphthalene-based polymers the number of thiophenes in the backbone has an influence on the bandgap, which is not seen for the benzene-based polymers. The bandgap is 0.11 eV, lower for JC6 compared to JC5. Fig. 5Absorption spectra of the six polymers in chloroform solution (top) and absorption spectra of films spin-coated on a glass slide from a chloroform solution (bottom, normalized). JC1 (brown), JC2 (black), JC3 (green), JC4 (yellow), JC5 (blue) and JC6 (pink). 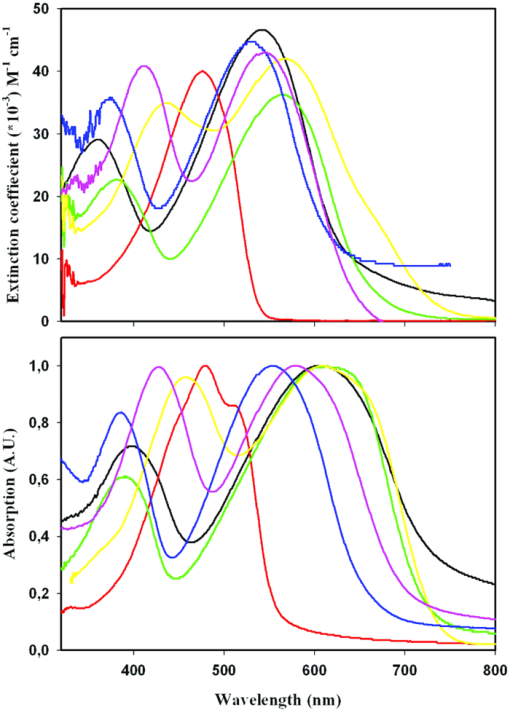 The absorption spectra shows that the π → π* transition for JC4 and JC6 are redshifted, both in solution and in film, compared to JC2, JC3, and JC5. This is due to extra thiophenes in the repeating unit. This shows that incorporation of BT units in the polymer results in a blueshift of the π → π* transition, but by increasing the number of donor units, here thiophenes, this can be shifted toward longer wavelengths. Exchanging the dialkoxybenzene (JC3 and JC4) with dialkoxynaphthalene (JC5 and JC6) resulted in a blueshift of the entire absorption spectrum in both cases. The difference is most pronounced between JC3 and JC5, where the CT transition is blueshifted with 59 nm for JC5 in film. Therefore a higher bandgap is also seen for JC5 and JC6 compared to JC2 to JC4. 3.3.Photovoltaic DevicesPhotovoltaic devices were prepared of the bulk heterojunction type consisting of: glass/ITO/PEDOT:PSS/polymer:C60 PCBM /aluminum, as described in the experimental section. The devices showed low to modest efficiencies (η), 0.005 to 2.2%, see Table 2. Devices prepared from polymer JC5 gave very low short circuit current densities (Jsc) of 0.1 mA/cm2, an open circuit voltage (Voc) of 0.14 eV, and η of 0.005% under AM1.5 illumination. The results, when held against the results from the other polymers, indicated that the fabrication of the photovoltaic device in this example had failed and may not be representative for its function in photovoltaic devices. Table 2Photovoltaic parameters for selected devices with the structure glass/ITO/PEDOT/ polymer:C60 PCBM /Al.
The other polymers produced Jsc of 2.6 to 4.7 mA/cm2 and Voc of 0.55 to 0.67 eV, under AM1.5 illumination. The fill factors were from 29 to 52%, which gave efficiencies of 0.4 to 2.2%. The donor-only type polymer JC1 showed an efficiency of 0.4%, while the polymers with incorporated BT acceptor units had higher efficiencies, 0.6 to 2.2%. This is in accordance with what the absorption spectra shows, where JC1 only has one strong absorption peak and the polymers containing BT have two strong absorption peaks. It is therefore possible that they can harvest a greater part of the incoming sunlight. The Jsc raises from 2.6 mA/cm2 to 4.6 to 4.7 mA/cm2 and the efficiency is more than doubled. JC4 and JC6 that have bithiophene instead of thiophene generates a lower Jsc than both JC2 and JC3. This can be due to the difference in the absorption whereby JC2 and JC3 have a better coverage of the solar spectrum which increases the Jsc through an increase in the number of absorbed photons. The naphthalene containing polymers gave significantly lower efficiencies compared to those with benzene, mainly due to a much lower Jsc of 0.1 and 2.6 mA/cm2, but also a lower Voc of 0.14 and 0.55 eV was observed. JC6 showed much higher Jsc, Voc and η than JC5. This pronounced difference in efficiency cannot be explained by the differences in the absorption spectra or by the differences in molecular-structure or -weight. The very low efficiencies of JC5 can be explained by defects in the photovoltaic devices. 4.ConclusionTwo new conjugated polymers, JC5 and JC6, consisting of 1,5-bis(2-hexyldecyloxy)naphthalene, thiophene, and BT groups have been synthesized and tested for optical and photovoltaic properties. The polymers showed two distinct broad areas of absorption where the spectrum of JC6 was redshifted compared to JC5. JC5 and JC6 bandgaps of 1.86 and 1.75 eV, respectably, which is higher than the bandgaps for the polymers having benzene and BT incorporated (JC2 to JC4). The photovoltaic devices prepared from the polymers showed low Jsc and Voc which resulted in low efficiencies of 0.005% (JC5) and 0.6% (JC6). This is lower than the best benzene incorporated polymer that showed efficiency up to 2.2%. The reason behind the low efficiency of the naphthalene-based devices and especially JC5 is probably due to defects in the produced devices as there is nothing in the molecular structure or the absorbing spectrum that can explain it. AcknowledgmentsThis work was supported by the Danish Strategic Research Council (DSF 2104-07-0022) and by EUDP (j. nr. 64009-0050). ReferencesC. J. Brabec, N. S. Sariciftci, and J. C. Hummelen,
“Plastic solar cells,”
Adv. Funct. Mater., 11 15
–26
(2001). http://dx.doi.org/10.1002/1616-3028(200102)11:1<15::AID-ADFM15>3.0.CO;2-A Google Scholar
H. Spanggaard and F. C. Krebs,
“A brief history of the development of organic and polymeric photovoltaics,”
Sol. Energy Mater. Sol. Cells, 83 125
–146
(2004). http://dx.doi.org/10.1016/j.solmat.2004.02.021 Google Scholar
K. M. Coakley and M. D. McGehee,
“Conjugated polymer photovoltaic cells,”
Chem. Mater., 16 4533
–4542
(2004). http://dx.doi.org/10.1021/cm049654n Google Scholar
H. Hoppe and N. S. Sariciftci,
“Organic solar cells: An overview,”
J. Mater. Res., 19 1924
–1945
(2004). http://dx.doi.org/10.1557/JMR.2004.0252 Google Scholar
C. Winder and N. S. Sariciftci,
“Low band gap polymers for photon harvesting in bulk heterojunctions solar cells,”
J. Mater. Chem., 14 1077
–1086
(2004). http://dx.doi.org/10.1039/b306630d Google Scholar
E. Bundgaard and F. C. Krebs,
“Low bandgap polymers for organic photovoltaics,”
Sol. Energy Mater. Sol. Cells, 91 954
–985
(2007). http://dx.doi.org/10.1016/j.solmat.2007.01.015 Google Scholar
S. Günes, H. Neugebauer, and N. S. Sariciftci,
“Conjugated polymer-based organic solar cells,”
Chem. Rev., 107 1324
–1338
(2007). http://dx.doi.org/10.1021/cr050149z Google Scholar
M. Jørgensen, K. Norrman, and F. C. Krebs,
“Stability/degradation of polymer solar cells,”
Sol. Energy Mater. Sol. Cells, 92 686
–714
(2008). http://dx.doi.org/10.1016/j.solmat.2008.01.005 Google Scholar
G. Li, V. Shrotriya, J. Huang, Y. Yao, T. Moriarty, K. Emery, and Y. Yang,
“High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blands,”
Nature Mater., 4 864
–868
(2005). http://dx.doi.org/10.1038/nmat1500 Google Scholar
W. Ma, C. Yang, X. Gong, K. Lee, and A. J. Heeger,
“Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology,”
Adv. Funct. Mater., 15 1617
–1622
(2005). http://dx.doi.org/10.1002/adfm.200500211 Google Scholar
J. Y Kim, K. Lee, N. E. Coates, D. Moses, T.-Q. Nguyen, M. Dante, and A. J. Heeger,
“Efficient tandem polymer solar cells fabricated by all-solution processing,”
Science, 317 222
–225
(2007). http://dx.doi.org/10.1126/science.1141711 Google Scholar
F. C. Krebs, J. Alstrup, H. Spanggaard, K. Larsen, and E. Kold,
“Production of large-area polymer solar cells by industrial silk screen printing, lifetime considerations and lamination with polyethyleneterephthalate,”
Sol. Energy Mater. Sol. Cells, 83 293
–300
(2004). http://dx.doi.org/10.1016/j.solmat.2004.02.031 Google Scholar
F. C. Krebs, H. Spanggaard, T. Kjær, M. Biancardo, and J. Alstrup,
“Large area plastic solar cell modules,”
Mater. Sci. Eng., B, 138 106
–111
(2007). http://dx.doi.org/10.1016/j.mseb.2006.06.008 Google Scholar
C. Lungenschmied, G. Dennler, H. Neugebauer, N. S. Sariciftci, M. Glatthaar, T. Meyer, and A. Meyer,
“Flexible, long-lived, large-area, organic solar cells,”
Sol. Energy Mater. Sol. Cells, 91 379
–384
(2007). http://dx.doi.org/10.1016/j.solmat.2006.10.013 Google Scholar
Y. Liang, Z. Xu, J. Xia, S. Tsai, Y. Wu, G. Li, C. Ray, and L. Yu,
“For the bright future—bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%,”
Adv. Mater., 22 E135
–E138
(2010). http://dx.doi.org/10.1002/adma.200903528 Google Scholar
Y. Kim, S. Cook, S. M. Tuladhar, S. A. Choulis, J. Nelson, J. R. Durrant, D. D. C. Bradley, M. Giles, I. McCulloch, C. S. Ha, and M. Ree,
“A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene: Fullerene solar cells,”
Nature Mater., 5 197
–203
(2006). http://dx.doi.org/10.1038/nmat1574 Google Scholar
R. Kroon, M. Lenes, J. Hummelen, P. Blom, and B. Boer,
“Small bandgap polymers for organic solar cells (polymer material development in the last 5 years),”
Polym. Rev., 48
(3), 531
–582
(2008). http://dx.doi.org/10.1080/15583720802231833 Google Scholar
M. Wienk, M. Struijk, and R. Janssen,
“Low band gap polymer bulk heterojunction solar cells,”
Chem. Phys. Lett., 422
(4–6), 488
–491
(2006). http://dx.doi.org/10.1016/j.cplett.2006.03.027 Google Scholar
J. E. Carlé, J. W. Andreasen, M. Jørgensen, and F. C. Krebs,
“Low band gap polymers based on 1,4-dialkoxybenzene, thiophene, bithiophene donors and the benzothiadiazole acceptor,”
Sol. Energy Mater. Sol. Cells, 94 774
–780
(2010). http://dx.doi.org/10.1016/j.solmat.2009.12.023 Google Scholar
N. C. Cates, R. Gysel, Z. Beiley, C. E. Miller, M. F. Toney, M. Heeney, I. McCulloch, and M. D. McGehee,
“Tuning the properties of polymer bulk heterojunction solar cells by adjusting fullerene size to control intercalation,”
Nano Lett., 9 4153
–4157
(2009). http://dx.doi.org/10.1021/nl9023808 Google Scholar
Q. Sun, K. Park, and L. Dai,
“Liquid crystalline polymers for efficient bilayer-bulk-heterojunction solar cells,”
J. Phys. Chem., 113 7892
–7897
(2009). http://dx.doi.org/10.1021/jp900241r Google Scholar
J. E. Parmer, A. C. Mayer, B. E. Hardin, S. R. Scully, M. D. McGehee, M. Heeney, and I. McCulloch,
“Organic bulk heterojunction solar cells using poly(2,5-bis(3-tetradecyllthiophen-2-yl)thieno[3,2,-b]thiophene),”
Appl. Phys. Lett., 92 113309
(2008). http://dx.doi.org/10.1063/1.2899996 Google Scholar
W. Tang, T. Kietzke, P. Vemulamada, and Z. Chen,
“Synthesis, characterization, and photovoltaic properties of novel conjugated copolymers derived from phenothiazines,”
J. Polym. Sci., Part A: Polym. Chem., 45 5266
–5276
(2007). http://dx.doi.org/10.1002/pola.22271 Google Scholar
J. Y. Lee, S. W. Heo, H. Choi, Y. J. Kwon, J. R. Haw, and D. K. Moon,
“Synthesis and characterization of 2,1,3-benzothiadiazole-thieno[3,2-b] thiophene-based charge transferred-type polymers for photovoltaic application,”
Sol. Energy Mater. Sol. Cells, 93 1932
–1938
(2009). http://dx.doi.org/10.1016/j.solmat.2009.07.006 Google Scholar
S. H. Mashraqui and H. Hariharasubrahmanian,
“Synthesis of 3,4Bis (4’Methylphenyl) thieno [2,3-b]Thiophene: First example of peri-diaryl thienothiophene,”
Synth. Commun., 30 1695
–1701
(2000). http://dx.doi.org/10.1080/00397910008087211 Google Scholar
M. Heeney, C. Bailey, K. Genevicius, M. Giles, M. Shkunov, D. Sparrowe, S. Tierney, W. Zhang, and I. McCulloch,
“Stable semiconducting thiophene polymers and their field effect transistor characteristics,”
Proc. SPIE, 5940 594007
(2005). http://dx.doi.org/10.1117/12.614778 Google Scholar
A. Cornel and G. Kirsch,
“Efficient one pot preparation of variously substituted thieno [2,3-b] thiophene,”
J. Heterocycl. Chem., 38 1167
(2001). http://dx.doi.org/10.1002/jhet.5570380521 Google Scholar
N. Hergué and P. Frère,
“Synthesis of 3,4-alkoxythieno[2,3-b]thiophene derivatives. The first block copolymer associating the 3,4-ethylenedioxythieno[2,3-b]thiophene (EDOThT) unit with 3,4-ethylenedioxythiophene (EDOT) moieties,”
Org. Biomol. Chem., 5 3442
–3449
(2007). http://dx.doi.org/10.1039/b712052d Google Scholar
G. Barbarella, M. Zambianchi, R. Di Toro, M. Colonna, D. Iarossi, F. Goldoni, and A. Bongini,
“Regioselective oligomerization of 3-(Alkylsulfanyl)thiophenes with ferric chloride,”
Org. Chem., 61 8285
–8292
(1996). http://dx.doi.org/10.1021/jo960982j Google Scholar
V. Niemi, P. Knuuttila, J. Österholm, and J. Korvola,
“Polymerization of 3-alkylthiophenes with FeCl3,”
Polymer, 33 1559
–1562
(1992). http://dx.doi.org/10.1016/0032-3861(92)90138-M Google Scholar
|