|
|
1.IntroductionSince the discovery of photoinduced electron transfer between semiconducting conjugated polymers and fullerenes, several investigations were made.1 Bulk heterojunction plastic cells (BHJs) based on composites of semiconducting polymers (as electron donors) and fullerene derivatives (as electron acceptors) were studied for their performances. The BHJ devices exhibit a vast interfacial area dispersed throughout the bulk. However, the overall conversion efficiency of these devices is limited by the carrier collection efficiency, which is greatly influenced by the morphology and the phase separation within the active film.2, 3, 4 Generally the morphology optimization of the active layers is of primary importance to improve the exciton dissociation efficiency, the transport of free charge carriers, and their extraction from the photoactive layer. To date, there were not many studies made on the functionalization of C70 for the photovoltaic (PV) application besides those already published on the PC70BM and the PC85BM. Using PC70BM into a photoactive layer increases the η% of the cells in both BHJ samples and tandem cases where it was permitted to reach 6%. In 2008, Heeger and co-workers obtained a 5.6% conversion using a photoactive layer based on PC70BM and the low bandgap polymer PCPDTBT.5 Improved photovoltaic properties were obtained for solar cells based on copolymer {thieno[3,4-b]thiophene and benzodithiophene polymers (PTBs)} by Luping Yu's team. The broad absorption of this copolymer increased the short circuit current density (JSC) of cells to 14.5 mA/cm2 with power efficiency around 7.4%.6 In our previous work, organic solar cells elaborated from the cyclopropano[60]fullerenes,7, 8 provided promising PV performances, with power conversion and current density above 1.2% and 7.2 mA/cm2, respectively. The effects of molecular structure on morphology and PV characteristics of RR-P3HT {poly (3,5 Hexylthiophene): cyclopropano[60]fullerenes} were studied. Along the same lines, cyclopropano[70]fullerenes were synthesised in order to study PV characteristics, keeping in mind that this family of fullerene C70 derivatives has a broader absorption in the visible region (see Fig. 1). One should note that this Bingel type approach of fullerene C70 functionalization is original, and was never studied before. 2.Experimental SectionCompounds C70-A, C70-B, and C70-C and C70 were analyzed in 7 × 10−4 mol l−1 dichloromethane (CH2Cl2) (HPLC grade) solutions containing 5 × 10−2 mol l−1 n-Bu4NPF6 as a supporting electrolyte. Electrochemical experiments were performed in a dry, oxygen-free (<0.1 ppm), argon glove box at room temperature with a platinum working electrode. Electrochemical experiments were carried out with an EGG PAR 273A potentiostat-galvanostat. Reduction potential was measured using a silver wire pseudo-reference electrode in reference with internal standard ferrocenium ion/ferrocene (Fc+/Fc) redox couple. The scan rate used in this study was 100 mV/s. Absorption spectra were recorded at room temperature with a Lambda 19 Perkin–Elmer spectrometer. These measurements were done on thin films based on composite P3HT: C70 fullerene derivatives. The substrates PEDOT:PSS {Poly(3,4 ethylenedioxythiophene): poly(styrenesulfonate)} coated ITO anode from Merck were first dried in an oven at 150°C during 10 min. The blend of RR-P3HT (Rieke): fullerene derivatives was dissolved in chlorobenzene solution. An active layer of RR-P3HT:cyclopropano[70]fullerene was deposited by spin coating over the PEDOT: PSS from Baytron P. Therefore, the LiF(Aldrich)-Al (Goodfellow) cathode was deposited by evaporation under a 4×10−7 mBar argon pressure, on top of the photoactive layer.9 The size of the active area is 0.32 cm2. The cells ITO/PEDOT: PSS(40 nm)/RR-P3HT:cyclopropano[70]fullerene(80 nm)/LiF(0.7 nm)/Al(100 nm) were characterized and annealed in the glove box. The PV measurements were carried out before and after heat treatment. The PV properties were measured using a Keithley 236 unit in the dark and under AM 1.5 illumination with 575 Steuernagel solar simulator, through the back side (ITO) of the solar cells. The conversion efficiency η% is calculated using: [TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation}
\eta \% = ({\rm ff}^\ast J_{{\rm sc}}^ \ast V_{{\rm oc}})/P_{{\rm inc}},
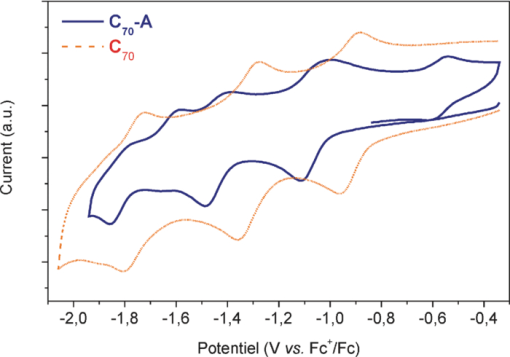
\end{equation}\end{document} where ff is the fill factor, Jsc is the short circuit current, Voc is the open circuit voltage, and Pinc is the incident light intensity.The external quantum yield or incident photon-to-electron conversion efficiency (IPCE) was measured using an Acton Spectra Pro 150 apparatus, by illuminating the solar cells with monochromatic light through the ITO. The relation between IPCE, current Isc, monochromatic intensity Iinc, and wavelength λ is given by: 3.Results and DiscussionThe electrochemical behavior of cyclopropano[70]fullerene derivatives and parent C70 as reference, has been investigated by cyclic voltammetry at room temperature in CH2Cl2 solution using TBAPF6 as the supporting electrolyte. The corresponding data are listed in Table 1. All derivatives show very similar voltammograms. The curves displayed by compound C70-A and C70 have been chosen as an example (see Fig. 2). Table 1Redox potentials of compounds C70-A, C70-B, and C70-C.
The voltammogram clearly shows 3 one-electron fullerene-based reduction waves at potentials E11/2 = –1.07 V, E21/2 = –1.44 V, and E31/2 = −1.82 V (half-wave potential versus Fc+/Fc), which are assigned to the generation of the anion radical C70•− , the dianion C70•2−, and the trianion C70•3− , respectively, from the cyclopropanoC70 moiety. That is characteristic of the electrochemical behavior of cyclopropano[70]fullerene issued from the Bingel process.10, 11, 12 Table 1 shows that all fullerene derivatives C70-A, C70-B, and C70-C are characterized by almost identical reduction potentials. It can thus be inferred that the differences observed in the structures of these derivatives, i.e., the ester chain length and/or the overall symmetry or nonsymmetry of the molecule, brings no net influence on the reduction ability of the compounds. On the other hand the redox properties of all cyclopropano[70]fullerenes appear to be close to those of parent C70. Table 2 shows a 70 mV negative shift for the first reduction step of the derivatives of C70-A, C70-B, and C70-C compared to the parent C70. This shift can be reasonably attributed to a partial loss of conjugation and alteration of the electron-accepting ability of C70 core further to LUMO energy elevation. These results can be explained by the presence of two –CO2R groups connected to the cyclopropano ring in C70-A, C70-B, and C70-C compounds. These ester groups are electron acceptors, which obviously increase the reduction potential of the C70 core in both series. The fourth oxidation wave at 0.59 V in the case of C70-A was attributed to the Cyclopronano moiety on C70.10 Table 2Relative quantities in mass of derivatives C70-A, C70-B, and C70-C used in photoactive layer.
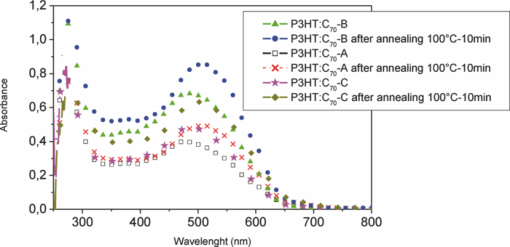
The UV-visible absorption spectra of the mixtures deposited as thin films are presented in Fig. 3. We notice that the absorption spectra have the same shape, with the same bands characteristic of the P3HT and C70 derivatives, before and after annealing. Heat treatment improves absorption coefficient of the samples. It involves a 30-nm shift of the spectra toward the highest wavelengths and an increase in absorption intensity with the appearance of shoulders at 565, 610, and 620 nm. This is usually attributed to the crystallization of RR-P3HT, further to phase separation. Although the molar concentrations of the fullerene derivatives are identical to those in the PCBM {[6,6]-phenyl-C61-butyric acid methyl ester} cell, PV characteristics delivered by the solar devices are not the same. That confirms the dependence of PV performances on the exact molecular structure and more precisely on the difference or similarity between ester side chains. The electrochemical characteristics showed that these derivatives have very comparable electronic properties. The PV characteristics strongly depend on the nature of the interaction between the donor and the acceptor, and on their behavior inside the active layer. However, molecular structure influences the PV parameters as well as η%. Indeed, the best performances are obtained from cells based on the composite P3HT: C70-B, with Jsc 3.56 mA/cm2, Voc 0.607 V, and ff 0.332 (Table 3). These parameters are improved after annealing at 100°C during 10 min (see Fig. 4). These parameters are smaller than in the P3HT:PC70BM system already studied by Yamanari;13 their cells provided a conversion efficiency of 3.8%. Table 3PV parameters of solar cells based on composites RR-P3HT: cyclopropano[70]fullerenes.
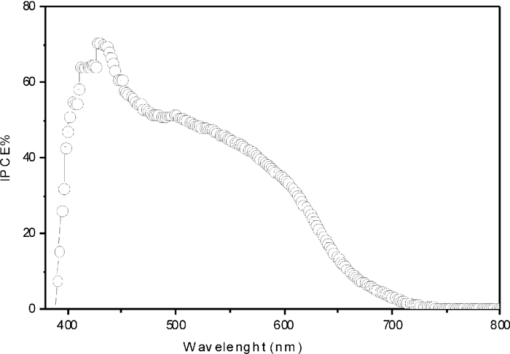
Fig. 4Current density/voltage (I–V) characteristic of cells based on composites RR-P3HT: cyclopropano[70]fullerene derivatives. 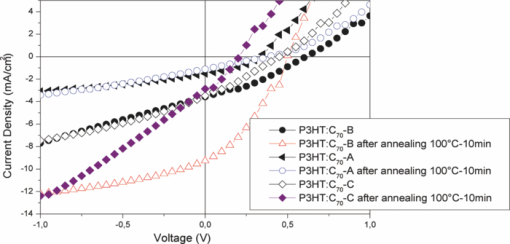 The heating effect causes phase separation and crystallization of the polymer RR-P3HT which allows the improvement of the absorption of the organic layers. Crystallization of the RR-P3HT increases the mobility of the charge carriers and thus we get Jsc = 9.29 mA/cm2 and ff = 0.342. Figure 5 shows a new peak around 430 nm with 71% IPCE which justifies the large Jsc. On the other hand, Voc slightly decreases. It can be caused by interface modification created during annealing. η% reaches 1.5% in that kind of solar cell. 4.ConclusionsIn the development of BHJ solar cells containing new fullerenes derivatives, it is clear that optimization alone of electronic and optical properties is insufficient.14, 15, 16 It is essential to take into account the molecular structure of the materials used, which can influence the interaction between donor and acceptor. These compounds C70-A, C70-B, and C70-C put in evidence the importance of the acceptor chemical structure on PV characteristics. They confirm also that synthesis strategies more direct than for the PCBM molecules are promising.7 The higher η% = 1.5% is obtained for devices based on C70-B after heat treatment at 100°C during 10 min. AcknowledgmentsWe are grateful to the ANR Nanorgysol, ORGAPVNET, and Département Maine et Loire for supporting our work. Work at Queen's was supported by NSERC, CFI, and CRC. ReferencesN. S. Sariciftci, L. Smilowitz, A. J. Heeger, and F. Wudl, Science, 258 1474
–1476
(1992). http://dx.doi.org/10.1126/science.258.5087.1474 Google Scholar
M. Reyes-Reyes, K. Kim, and D. L. Carolla, Appl. Phys. Lett., 87 083506
(2005). http://dx.doi.org/10.1063/1.2006986 Google Scholar
W. Ma, C. Yang, X. Gong, K. Lee, and A. J. Heeger, Adv. Funct. Mater., 15 1617
–1622
(2005). http://dx.doi.org/10.1002/adfm.200500211 Google Scholar
G. Li, V. Shrotriya, Y. Yao, and Y. Yang, J. Appl. Phys., 98 043704
(2005). http://dx.doi.org/10.1063/1.2008386 Google Scholar
I. W. Hwang, S. Cho, J. Y. Kim, K. Lee, N. E. Coates, D. Moses, and A. J. Heeger, J. Appl. Phys., 104 033706
(2008). http://dx.doi.org/10.1063/1.2951957 Google Scholar
Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray, and L. Yu, Adv. Mater., 22 E135
–E138
(2010). http://dx.doi.org/10.1002/adma.200903528 Google Scholar
H. Derbal, C. Bergeret, N. Ziani, J. Cousseau, and J. M. Nunzi, 42
(2007). Google Scholar
H. Derbal, C. Bergeret, J. Cousseau, and J.-M. Nunzi, 932
–935
(2009). Google Scholar
S. Alem, R. de Bettignies , J.-M. Nunzi, and M. Cariou, Appl. Phys Lett, 84 2178
–2180
(2004). http://dx.doi.org/10.1063/1.1669065 Google Scholar
M. Oçafrain, M. A. Herranz, L. Marx, C. Thilgen, F. Diederich, and L. Echegoyen, Chem. Eur. J., 9 4811
–4819
(2003). http://dx.doi.org/10.1002/chem.200304935 Google Scholar
J.-F. Eckert, J.-F. Nicoud, J.-F. Nierengarten, S.-G. Liu, L. Echegoyen, F. Barigelletti, N. Armaroli, L. Ouali, V. Krasnikov, and G. Hadziioannou, J. Am. Chem. Soc., 122 7467
–7469
(2000). http://dx.doi.org/10.1021/ja9941072 Google Scholar
J. Li, N. Sun, Z.-X. Guo, C. Li, Y. Li, L. Dai, D. Zhu, D. Sun, Y. Cao, and L. Fan, J. Phys. Chem. B, 106 11509
–11514
(2002). http://dx.doi.org/10.1021/jp025973v Google Scholar
T. Yamanari, T. Taima, J. Sakai, and K. Saito, Jpn. J. Appl. Phys., 47 1230
–1233
(2008). http://dx.doi.org/10.1143/JJAP.47.1230 Google Scholar
L. Sicot, C. Fiorini, A. Lorin, P. Raimond, C. Sentein, and J. M. Nunzi, Sol. Energy Mater. Solar Cells, 63 49
–60
(2000). http://dx.doi.org/10.1016/S0927-0248(00)00019-2 Google Scholar
J. M. Nunzi, C. R. Acad. Sci. Paris, 3 523
–542
(2002). http://dx.doi.org/10.1016/S1631-0705(02)01335-X Google Scholar
A. K. Pandey, S. Dabos-Seignon, and J. M. Nunzi, Appl. Phys. Lett., 89 113506
(2006). http://dx.doi.org/10.1063/1.2352800 Google Scholar
BiographyHassina Derbal-Habak received her PhD at the University of Angers, France in 2009. She made several national and international collaborations. In September 2009, she joined IM2NP laboratory in the “Optoelectronics and photovoltaics components” team as POST-DOC. Her main research interests are photonics, organic solar cells, organic components, bulk nanostructured and nanoplasmonic structures for PV. Celine Bergeret received her PhD at the University of Angers, France in 2009. Her main research interests are the developments of modified fullerenes and carbon nanotubes for organic components and supramolecular structures. She is involved in the setup of new plastic solar cells. Jean Jacques Simon received his PhD at the University of Marseille in 1996 for a thesis on “Electrical activity of dislocations in silicon.” From 1997 to 2000, he worked as a process engineer for IBM Semiconductor at Corbeil-Essonnes, France. He joined the University Paul Cezanne of Marseille in 2000 as a senior lecturer and for his research the Fresnel Institute in the group “Microstructured Optical Components.” He joined the IM2NP laboratory in 2007 and his current research interests are photonics for organic and thin film solar cells. He has authored more than 30 papers and communications. Ludovic Escoubas is Professor at the Aix Marseille University and leader of the OPTO-PV group at the Materials, Microelectronics and Nanoscience Institute of Provence (IM2NP). His current research interests are photonic structures in organic solar cells and surface structuration of components for light propagation control. He has authored more than 50 papers in peer-reviewed scientific journals and holds 7 patents. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||