|
|
1.IntroductionOptoelectronic devices based on organic semiconductors, including conjugated small molecules and polymers, have attracted tremendous academic and industrial attention, due to the many technological advantages of organic materials, such as low material cost, great diversity, tunable material properties, and compatibility with flexible substrates and low temperature/high throughput manufacturing processes. 1 – 4 Over the last decade, the performance of optoelectronic devices has rapidly advanced in terms of efficiency and lifetime, especially those of organic photovoltaic (OPV) cells and organic light-emitting diodes (OLEDs). The state-of-the-art power conversion efficiency of OPV devices has exceeded 10%, which is now approaching the proposed industry required efficiency. 5 , 6 The OLED technology has already reached commercial applications in displays: Samsung’s active-matrix OLED displays in smart phones and LG’s 55-in. OLED TV are some examples. As shown in Fig. 1, the basic device architectures of most OPV and OLED devices can be classified into three different groups according to the position of the light transmissive window, i.e., the transparent electrode (TE), which allows light into and out of the devices, which also relates to the device fabrication sequence. Most commonly, as shown in Fig. 1(a), a TE is first deposited on a transparent substrate, upon which organic layers and a reflective electrode are subsequently deposited. This configuration corresponds to the superstrate type for OPV devices or the bottom-emitting type for OLEDs, in which light travels through the bottom transparent electrode and the substrate. When the positions of the transparent and reflective electrodes in the devices are switched as shown in Fig. 1(b) such that light travels through the top electrode, we have a substrate type OPV device or a top-emitting OLED. Here, an opaque substrate could be used. In some other cases, transparent (or semitransparent) OPV or OLED devices can be achieved when both electrodes are transparent (or semitransparent) such that light travels through the bottom and top electrodes as shown in Fig. 1(c). Fig. 1Schematic illustration of three types of general organic photovoltaic (OPV) and organic light-emitting diode (OLED) device architectures: (a) superstrate type OPV and bottom-emitting OLED, where light transfers into or out of the device through bottom substrates and transparent electrodes; (b) substrate type OPV and top-emitting OLED, where light travels through top transparent electrodes; and (c) transparent OPV and OLED, where light travels through both bottom and top transparent electrodes. 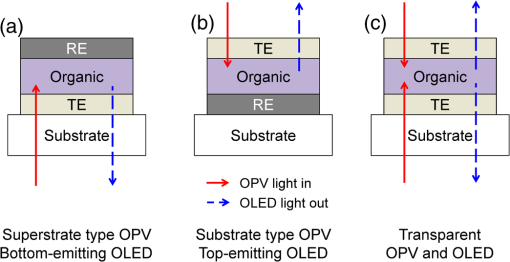 As a TE in optoelectronic devices, optical transparency and electrical conductivity are obviously two key parameters that are related to the device performance. Moreover, the electrode/active layer interfaces, which can strongly influence the device performance, are closely related to various material properties of the electrodes and the deposition methods used. Hence, other properties, such as surface roughness, surface chemistry, work function, processibility, and mechanical properties, are also important factors for evaluating a candidate material for use as the TE in a particular type of device. Some of these parameters are especially important when the transparent electrodes are deposited on top of the organic layers [see Figs. 1(b) and 1(c)]. As organic materials are generally susceptible to high temperature, organic solvents, and ion/atom bombardment, it is more challenging to find suitable top transparent electrodes and related deposition techniques that can eliminate or minimize the damage to underlying active materials. So far, indium-tin oxide (ITO) has been the material of choice as the TE in most OPV and OLED devices as a result of its excellent optoelectronic properties and environmental stability. 7 – 9 Commercial ITO thin films usually have sheet resistances of or less for 100- to 300-nm-thick films (corresponding to conductivity on the order of ) and a typical transmittance of in the visible spectral range. Although ITO is the dominant electrode for optoelectronic devices, it still has several drawbacks for large-scale applications. 10 – 12 First, the rising cost of indium, associated with the scarcity of global indium resources and its large consumption, is one of the greatest concerns for the boosted demand of ITO due to the popularity of liquid crystal displays, solid-state lighting, solar panels, and other products. 13 – 15 The deposition techniques and surface treatment processes also add to the cost of ITO. 16 Generally, most of the commercialized ITO thin films are deposited by magnetron sputtering, molecular beam epitaxy, thermal evaporation, and pulse laser deposition. All these techniques require high treatment temperatures, 400 to 500°C or higher, and high-vacuum related deposition tools, which further increases the ITO price 17 , 18 and makes it unsuitable for use as top electrodes in organic optoelectronic devices or for use with plastic substrates. Furthermore, the inefficient material utilization for these deposition techniques is another factor that increases the cost of ITOs. For example, of the ITO in the source targets can reach the substrates during the sputter deposition process, and the remaining 70% of the sputtered ITO source material is left on the sidewalls and masks of the sputtering chamber. ITO recycling, either from the deposition process or from the scraped optoelectronic products containing ITO films, has become one of the critical ways to minimize ITO manufacturing costs. Aside from the high cost of raw materials, the high temperature-related deposition processes will damage the underlying organic materials when being utilized as top TEs in OPV and OLED devices. Due to the intrinsic flexibility of organic semiconductors, the next-generation organic optoelectronic devices are envisioned to be fabricated on flexible substrates to realize flexible and lightweight optoelectronic products. However, the high temperature deposition process and the brittle nature of ITO impede its applications in flexible modules. Last but not least, the nonstoichiometry nature of ITO makes its properties very variable and strongly dependent on the processing history (including source material composition, deposition method and conditions, postdeposition handling/treatment). 9 In the past few years, various alternative transparent conductive materials have been developed to address the above issues of ITO. In this review, we discuss four groups of materials that have been utilized as TEs in organic optoelectronic devices, especially in OPV and OLED devices: doped metal oxides, thin metal layers, transparent conductive polymers, and nanoscale materials [carbon nanotubes (CNTs), graphene, and metal nanowires (NWs)]. High optical transparency in designated spectral regions and low sheet resistance are generally required to achieve high optoelectronic device performance; however, there generally exists a trade-off between these two key parameters. If we neglect any surface or interface effects and assume the material is homogeneous, the sheet resistance of the electrode, , should scale inversely with its thickness, , i.e., , whereas the transparency, , follows an exponential decay behavior with as according to the Beer-Lambert law. Here and are the electrical conductivity and absorption coefficient (which is wavelength dependent), respectively. Hence increasing the electrode thickness leads to lowering in both sheet resistance and transparency. In addition to the thickness dependency, the transparency of these electrodes could have a weak or strong wavelength dependence, and the surface/interface effects (roughness, patterning, optical interference, etc.) as well as material synthesis/processing conditions could also play important roles in determining both transparency and resistance in many cases. The representative transparency (in the visible spectral range) and sheet resistance values of the four groups of transparent conductive materials in optimized (or near-optimized) conditions are summarized in Fig. 2 and Table 1. 11 , 13 , 19 – 53 Table 1 also summarizes some other relevant factors for using these materials as TEs in OPV and OLED devices/modules, which include compatibility with flexible substrates and large-scale processes, the possibility of using them as top TEs, and their environmental stability. Fig. 2Literature-reported transparency values in the visible spectral range as a function of the sheet resistance for transparent conductive materials: indium-tin oxide (ITO), 22 , 35 other doped metal oxides, 21 , 33 thin metal layers, 29 , 34 , 41 , 46 dielectric/metal/dielectric multilayers, 44 metal grids, 23 , 52 poly(3,4-ethylene dioxythiophene):polystyrene sulfonic acid layers, 13 , 15 , 36 , 37 , 45 , 48 , 51 graphene, 28 , 29 , 31 , 32 , 47 carbon nanotube films, 19 , 20 , 26 , 30 , 43 , 45 , 53 and Ag nanowires. 11 , 29 , 39 , 40 , 42 , 50 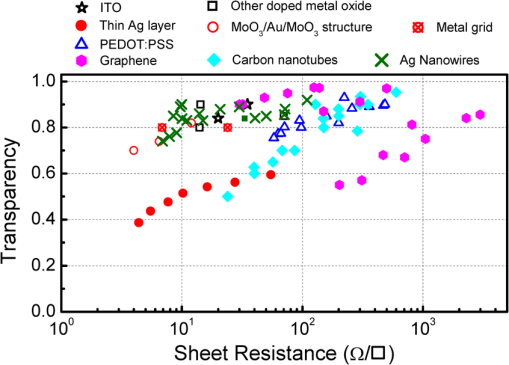 Table 1List of representative key parameters of different types of transparent conductive materials for use as transparent electrodes in organic optoelectronic devices.
In the following sections, we will review the basic material/film properties for all four groups of materials in detail, along with their compatibility with large-scale and low-cost fabrication methods. We will also review their applications in OPV and OLED devices, and will especially compare the device performance and device flexibility based on these alternatives with ITO-reference devices then discuss their possibility as top TEs. 2.Transparent Conductive OxidesDoped metal oxides are the most common transparent conductive oxide layers in organic optoelectronic devices. Doped metal oxide thin films are usually highly transparent ( transmittance) due to the wide bandgap of most metal oxides, while the doped elements can generate oxygen vacancies, interstitial defects, and doped metal ions that assist the charge carrier transport and enable the high conductivity of oxide films. 54 , 55 As mentioned above, ITO is the most widely used TE material in OPV and OLED devices with its excellent electronic conductivity and optical transparency in the visible light range. In most OPV and OLED devices, ITO is usually deposited on transparent substrates as the bottom electrode and light window; therefore, the physical properties of ITO films, including the surface roughness, film conductivity, work function, etc., strongly affect the device performance. Extensive work has been carried out to modify the ITO surface with various treatments, such as mechanical polishing, ion-beam sputtering, oxygen plasma, and UV-ozone or chemical treatments. 9 , 56 , 57 The work function (WF) of an electrode plays a critical role in determining the carrier (electron or hole) injection barrier height into the active materials. The WF of as-received ITO films is typically in the range from 4.3 to 4.7 eV, which is neither close to the lowest unoccupied molecular orbital (LUMO) level nor to the highest occupied molecular orbital level of most organic semiconductors used in OLED and OPV devices. Hence, the nonzero potential barrier for the hole or electron states between the ITO electrodes and organic layers may present a critical limit on the charge collection/injection efficiency for OPV and OLED devices. 58 – 60 Several surface treatments have been demonstrated to modify the work function for achieving a better device performance. UV-ozone and plasma treatment are the most simple and widely used methods to increase the work function of ITO films, normally up to 4.7 to 5.0 eV, which is generally attributed to the incorporation of oxygen onto the ITO electrode surface. 61 , 62 The solution casted poly(3,4-ethylene dioxythiophene):polystyrene sulfonic acid (PEDOT:PSS) layer ( ) is the most popular anode modification layer for OPV and OLED devices with ITO as anodes. 63 , 64 Alternatively, some transparent transition metal oxide thin films have been demonstrated as good buffer layers between ITO and organic layers. Normally, , NiO, and are hole-selective materials in optoelectronic devices and are used as anode modification materials, 65 – 68 whereas ZnO and are electron-selective layers that allow efficient electron collection and injection. 69 – 73 Self-assembling a monolayer of organic molecules on the ITO surface has emerged as another effective routine to modify the electronic and chemical properties of the ITO surface and engineer the ITO/organic interfaces. In general, one end of these self-assembled molecules can spontaneously form tight bonds with the surface atoms of ITO, whereas the other end of the molecular chain can be functionalized with polar groups. The dipole moments of these molecules will affect the vacuum level outside the ITO electrode and vary its effective work function. 74 – 77 Recently, Helander et al. reported another novel method to significantly increase the ITO work function by functionalizing the ITO surface with a monolayer of chlorine [named chlorinated ITO (Cl-ITO)]. With a controllable number of electron-negative halogen atoms anchored on ITO surface, the ITO work function can be tuned from 4.7 eV to a maximum value of . 78 Helander et al. also demonstrated phosphorescent OLED devices with the Cl-ITO anodes, where the high ITO work function matched with the LUMO level of the host material, 4,4′-N,N′- dicarbazole-biphenyl, and enabled the direct injection of holes from ITO to the host. Therefore, the OLED device structure was simplified with no hole injection and transport layers and the device efficiency was greatly improved with less charge transport barriers and exciton quenching interfaces. 78 Magnetron sputtering, molecular beam epitaxy, thermal evaporation, pulse laser deposition, etc., are the major deposition techniques for achieving high-quality ITO films. Normally, these techniques are suitable for ITO deposition on hard substrates that can withstand high temperature processing as bottom electrodes in OPV and OLED devices; however, the high-energy ions or atoms along with possible heat and radiations during these deposition processes may cause significant damage to the underlying organic films, thus limiting the application of ITO electrodes as top electrodes in organic-based optoelectronic devices. 79 , 80 For example, sputtering ITO without specifically heating the substrate often leads to lower electrical conductivity and/or lower optical transparency than in commercially available ITO electrodes on glass substrates, which generally require high substrate temperatures during deposition or high temperature postdeposition annealing. 9 When using ITO as the top electrode, it is desirable to use some buffer layers or sacrificial layers, such as organic protective layers [e.g., copper phthalocyanine (CuPc) layer], 81 thin metal layers (e.g., Mg/Ag layer), 82 or some transition metal oxide layers (e.g., ), 83 to protect the underlying organic active layer during ITO deposition, without negatively affecting the charge injection/transport characteristics of the device. Low-energy magnetron sputtering methods have also been developed to reduce the damages to organic layers. 84 – 86 There have been several successes in demonstrating OPV and OLED devices with sputtered top ITO electrodes; however, the optical and electronic properties for these ITO films with low energy deposition techniques are quite different from commercial ITO electrodes in addition to a very slow deposition process with film growth rates as low as , 9 , 87 which need to be further studied and improved for their large-scale applications. Furthermore, the poor mechanical properties of ITO layers are another issue that affects their application in flexible and portable electronic products. As reported in previous literature, microcracks were observed in ITO thin layers after repeated flexing and large angle bending, leading to a dramatic decrease in film conductance as well as device performance. 44 , 48 , 88 Though there are companies and research groups working on methods to engineer the ITO film quality, such as varying the deposition methods and modifying the indium to tin ratio, it is still a great challenge to fully solve this problem. Zinc oxide (ZnO), which has a wide bandgap ( ) and is transparent in the visible spectral range, is one of the attractive ITO alternates due to the abundant material storage and its environmental nontoxicity. Similar to that of ITO, some extrinsic dopants, such as Al and Ga, are added into the ZnO films to improve the film conductivity by introducing ionic impurities. 33 , 45 , 89 Hence, these Al-doped ZnO (AZO) 33 , 90 , 91 and Ga-doped ZnO (GZO) 21 , 92 films show comparable optical transparency and electrical conductivity as the ITO layers and have been developed as TEs in optoelectronic devices. AZO and GZO have a work function range of 4.0 to 5.0 eV depending on the surface treatments. 93 , 94 By selecting suitable interlayers as those described for ITO modification, they can serve as either anodes or cathodes in OPV and OLED cells. For example, Liu et al. reported an -thick AZO electrode with a sheet resistance of and a transparency of in the visage spectral range. An ITO-free inverted poly[[4,8-bis[(2-ethylhexyl)oxy]benzo-[1,2-b:4,5- ]dithiophene-2,6-diyl][3-fluoro-2-[(2-ethylhexyl)-carbonyl]-thieno-[3,4- ]-thiophenediyl]]:[6,6]-phenyl- -butyric acid methyl ester OPV device was successfully demonstrated with such an AZO TE, which showed an optimized power conversion efficiency ( ) of 6.15%, only slightly lower than the ITO-based devices ( ). 91 Other than AZO and GZO, many other doped metal oxides have been investigated and utilized as TEs in optoelectronic devices. For example, fluorine-doped tin oxide is another type of the doped metal oxide electrodes that are widely used as electron collection electrodes in dye sensitized solar cells. 95 , 96 Most recently, they have also been broadly used as bottom transparent cathodes in inverted planar heterojunction solar cells due to the rapidly developing new photovoltaic active materials, perovskites, with a power conversion efficiency of . 96 – 98 Though the material-related cost for these doped metal oxide is reduced by getting rid of the scarce materials, similar costly deposition techniques, such as sputter, chemical vapor deposition, atomic layer deposition, and pulse laser deposition, are still required for achieving high-quality films for such indium-free materials. 89 , 99 More efforts are required to develop cheaper processing methods (e.g., solution-process) to reduce the overall cost for TE fabrication. As ceramics, they also have poor mechanical properties like ITO, which is not compatible with flexible substrates and high-throughput roll-to-roll processes. Additionally, some reports have shown that some of these materials like AZO have poor environmental stability when they are ultrathin ( ), further limiting their applications in optoelectronic devices. 24 3.Metal-Based (Semi-)Transparent ElectrodesWhile thick metal layers (e.g., Al and Ag), with thicknesses on the order of 100 nm, have been widely used as reflecting electrodes in most optoelectronic devices, ultrathin metal layers (normally thick) are semitransparent to visible light due to the skin depth of the electromagnetic wave for these metals. Such ultrathin metal layers have emerged as a potential ITO substitute in optoelectronic devices in laboratories. 100 – 104 Significant effort has been made to improve the transparency of ultrathin metal layers while maintaining their high conductivity. 105 In this section, we will discuss the application of thin metal layers, dielectric/metal/dielectric multilayer structures, and metal grids for improved transparency as TEs in OPV and OLED devices. 3.1.Ultrathin Metal LayersThere is a trade-off between the transparency and conductivity for ultrathin metal layers. Though a sufficiently thin metal layer allows most of the light to be transmitted (high transparency), the discontinuity in film morphology results in poor electrical conductivity for ultrathin metal films. 41 , 106 , 107 Wilken et al. measured the sheet resistance of Au thin films on glass substrates with different nominal film thicknesses (from 10 to 60 nm). As shown in Fig. 3(a), while the experimental data (open symbols) qualitatively follow the predicted trend from the Fuchs-Sondheimer and Mayadas-Shatzkes models based on the scattering of free charge carriers, 104 the Au films with thicknesses have low sheet resistances of ; however, the sheet resistance dramatically increases to for films . Shown in the inset of Fig. 3(a) is a comparison of transmittance of the Au films with different thicknesses. A maximum transmittance of at was obtained for a 7-nm-thick Au film, which was reduced to for a 12-nm-thick film. In addition, the transmittance exhibits strong wavelength dependence. For example, the transmittance is only 50% at compared to the peak of 70% at for the 7-nm-thick Au film. Fig. 3(a) Sheet resistance of ultrathin Au films with various thicknesses (open dots are experimental results and solid lines are the prediction based on Fuchs-Sondheimer and Mayadas-Shatzkes mode). Inset: Transmittance of Au films with differet thicknesses. Reprinted with permission from Ref. 104. Copyright 2012 Elsevier. (b) A comparison of characteristics of CuPc/C60 devices with ITO and Ag thin films as bottom transparent electrodes. Reprinted with permission from Ref. 103. Copyright 2008 AIP Publishing LLC. 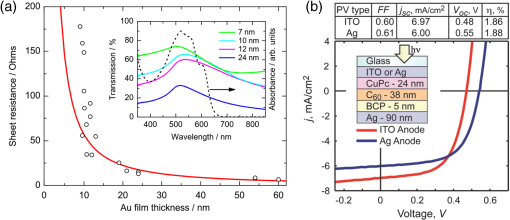 With a 9-nm-thick Ag layer as the bottom TE, O’Connor et al. successfully demonstrated a bilayer OPV device, which exhibited a similar PV performance to that with an ITO electrode [Fig. 3(b)]. 103 Although there have been a few studies aimed toward increasing the electrical conductivity without sacrificing the optical transparency, 46 , 108 , 109 such as applying a seed layer to improve the continuity of thin metal layers, 110 the relatively low optical transparency of thin metals is still one of the limitations in achieving high-performance devices with them. 111 , 112 3.2.Dielectric/Thin-Metal/Dielectric ElectrodesSince the first report by Fan et al. in 1974, 113 dielectric/thin-metal/dielectric (DMD) multilayer structures have been extensively studied for achieving highly transparent and conductive electrodes. 114 – 117 In these DMD electrodes, the intermediate thin metal layer provides the electrical conductance for the entire structure, whereas the two dielectric layers improve the overall transparency due to optical interference within the multilayer structure and the surface plasmonic effects at the two metal/dielectric interfaces. 44 , 118 – 120 As shown in Figs. 4(b) and 4(c), an structure shows a maximum transmittance of at (the corresponding reflectance is ), which is about two times higher than that of the bare Au layer ( and ). These experimental results qualitatively agree with the calculated transmittance and reflectance [open symbols in Fig. 4(b)] based on the transfer-matrix model with the optical constants listed in Fig. 4(a), which further confirms the transparency enhancement of the DMD structure as a result of the strong optical interference effects within the multilayer structure. 44 In addition to , 115 , 121 other choices for the dielectric layers include metal oxides (ZnO, 122 , 123 , 124 etc.), metal sulfides [e.g., ZnS (Refs. 125 and 126], and organic materials (bathocuproine). 127 For example, Cho et al. applied the electrode in OLEDs with tris(8-hydroxyquinolinato) aluminum ( ) active layers, which exhibited near-Lambertian emission with comparable current efficiency to ITO-based reference devices. 125 The inner dielectric layer in these DMD electrodes determines the carrier injection/collection polarity of the whole structure. Other than the rare and noble metal materials (e.g., Au and Ag), some other metals, such as Al and Cu, have also been utilized in the electrodes without affecting their electrical properties, which can further reduce the material cost for the entire structure. 128 All the layers in the DMD structures are deposited by low-temperature deposition techniques, such as thermal vacuum deposition and spin coating; hence, these DMD electrodes can be utilized as top TEs with negligible damage to the underlying organic layers. 115 , 116 , 129 Wrzesniewski et al. successfully demonstrated a top-emitting OLED (TE-OLED) with a thermal evaporated trilayer structure as the top TE, which enabled a very high light extraction efficiency. 116 With transparent and close-packed microlens arrays attached to the DMD electrode, the light extraction efficiency as well as the external quantum efficiency of such TE-OLED devices was increased by up to 2.6 times compared to the ones without the microlens arrays. 116 Fig. 4(a) Optical constants of thin layers of and Au for transfer-matix simulations. (b) Comparison of the transmittance of trilayer electrode and a 15-nm-thick Au thin layers. (c) Comparison of the reflectance of the trilayer and Au thin layers, where solid symbols are experimental results and open symbols are calculated results. Reprinted with permission from Ref. 44. Copyright 2012 Elsevier. 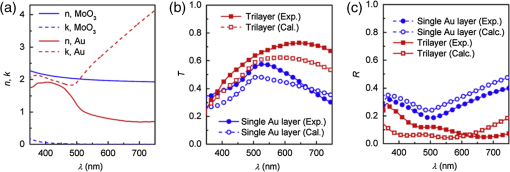 Furthermore, such DMD multilayer electrodes show excellent mechanical flexibility in comparison to ITO electrodes because of the good ductility of metal layers, enabling their application in flexible optoelectronic devices. 122 Cao et al. successfully demonstrated a flexible poly(3-hexylthiophene):phenyl- -butyric acid methyl ester (P3HT:PCBM) device with electrodes on poly(ethylene terephthalate) (PET) substrates [Fig. 5(a)], which shows a nearly identical photovoltaic performance as the similar device fabricated on a glass substrate [Fig. 5(b)]. Cao et al. further studied the mechanical flexibility of the flexible device using a simple bending test method. 44 Only an drop in efficiency was observed after bending the device 500 times with a bending radius of 1.3 cm [Fig. 5(c)], which is far better than ITO electrodes, which can only sustain a few bending cycles with large bending angles. 44 Fig. 5(a) A photograph of a flexible poly(3-hexylthiophene):phenyl-C61-butyric acid methyl ester (P3HT:PCBM) device with an transparent electrode. (b) A comparison of characteristics of P3HT:PCBM devices on glass and plastic substrates. (c) characteristics of devices under different bending conditions. Reprinted with permission from Ref. 44. Copyright 2012 Elsevier. 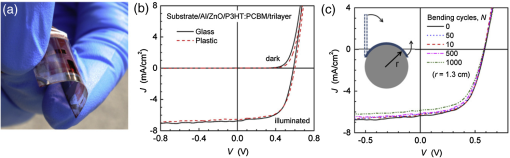 In summary, the multilayer structure with a thin metal layer sandwiched between two dielectric layers shows a similar electrical conductivity but an enhanced optical transparency compared to the metal-only semitransparent electrodes. With some advantages over ITO electrodes, such as better flexibility in flexible devices and less damage to organic layers when used as top TEs, these multilayer electrodes have been investigated as potential ITO substitutes. So far, sputter and thermal evaporation are the two major deposition methods for metal and dielectric layers in these DMD electrodes. Achieving the desired thickness for each layer in DMD structures may present some cost issues for fabricating low-cost optoelectronic devices. 130 Moreover, transparency and conductivity of the DMD layers are strongly dependent on the thickness of the multiple thin layers, especially the intermediate metal layer, which is only thick. Therefore, the uniformity of these ultrathin layers is vitally important for optimal properties; better deposition methods are required to make them compatible with large-area OPV and OLED modules. 3.3.Metal GridsMacroscopic metal grids have been widely utilized as the front contact in many inorganic-based solar cells, such as silicon solar cells and copper indium gallium selenide photovoltaic cells, where the metal fingers conduct away the generated current and the openings between the figures become the light windows for light absorption. 131 , 132 Here, microscopic metal grids composed of ordered metal lines that are wide are discussed and considered as a potential replacement for semitransparent continuous metal films for use as TEs in organic optoelectronic devices. 133 – 135 One of the advantages of metal grids is the increased transparency compared to continuous metal layers, where the gaps between metal lines are blank (100% in transparency) and contribute to the high transparency. The transmittance of metal grids is determined by the percentage of the blank areas on the film, which is related to the metal line width, spacing between lines, and the number of total lines within a unit area. The low sheet resistance of the metal grids can be achieved by using thick metal lines, 100 nm or even thicker, although this comes at the expense of film planarity. 52 However, the openings in the metal grids result in poor electrical contact with the organic materials, which typically have very low conductivity, thus leading to very inefficient charge injection and/or collection at the electrode/organic interface. Generally, a continuous conductive buffer layer (e.g., PEDOT:PSS) is required to planarize the surface of the metal grids and improve electrode contact with the active materials when used in optoelectronic devices. 136 Conventional photolithography has been one of the predominant patterning methods used to fabricate such ordered metal grids, although it is somewhat complicated, costly, and incompatible with flexible substrates. 137 , 138 In 2007, Kang and Guo demonstrated a nanoimprint lithography (NIL) technique to achieve these electrodes on both rigid and flexible substrates. 139 , 140 As schematically illustrated in Fig. 6(a), a soft mold (e.g., polydimethylsiloxane) is first created with the replication of desired features from a hard template patterned using conventional photolithography. The soft mold is a reusable transfer media or stamp which transfers a thin metal layer deposited on its surface to the substrates by applying proper pressure and heat. 139 , 141 Different metal grids, such as Au, Cu, and Ag, have been successfully fabricated with this NIL method. 23 These printed grids with 70-nm line width and 700 nm periods [Fig. 6(b)] showed an average transmittance of 84, 83, and 78% in the visible spectral range and sheet resistances of 24, 28, and for Au, Cu, and Ag electrodes, respectively [Fig. 6(c)]. With these metal grids as TEs, both ITO-free OPVs and OLEDs were successfully demonstrated with comparable device performance as the ITO-reference cells. 136 , 137 , 140 , 142 For example, an of was achieved by using the above metal grids as the transparent anodes in P3HT:PCBM devices, which is comparable to the ITO-based devices fabricated under the same conditions. 23 Fig. 6(a) Schematic illustration of a nanoimprint lithography (NIL) technique to produce metal grids. (b) SEM images of Cu grids fabricated by the NIL method. (c) Optical transmittance of Au, Cu, and Ag grid electrodes and a conventional ITO electrode. Reprinted with permission from Ref. 23. Copyright 2008 John Wiley and Sons. 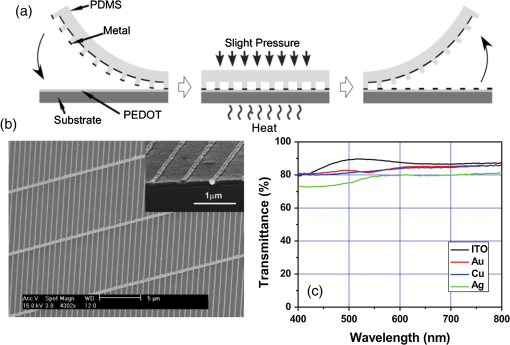 Although the development of the NIL technique presents a great opportunity for using metal grids as TEs in electronic devices, there are still several factors that influence the large-scale applications. The extremely rough surfaces (generally in the range of tens of nanometers) limit their application in devices with thin organic layers. It is also difficult to use such metal grids as top TEs in OPVs and OLEDs since the transfer processes (heat or pressure) may cause damage to the underlying organic layers. Moreover, more effort is needed to integrate the NIL techniques with roll-to-roll processing, which is another significant challenge for utilizing metal grids as TEs in large-scale devices. 4.Polymeric Transparent ConductorsDue to the high transparency, easy solution processability, and good compatibility with flexible applications, transparent conductive polymers have also been investigated as alternative low-cost TEs. 12 , 17 , 36 , 143 , 144 To achieve high electrical conductivities, some conductive polymers, such as polyaniline, polypyrrole, and polythiophene, have been developed with the addition of suitable chemical dopants. Among these, PEDOT:PSS is one of the most successful and widely used conducting polymers for optoelectronic applications, especially for those in organic-based devices. 37 , 51 , 145 Since its discovery in the 1990s, PEDOT:PSS has been widely used as an interlayer in organic-based electronic devices, such as polymer PVs and LEDs, to reduce the ITO roughness and facilitate the hole collection/injection between the polymers and the ITO. 146 Low conductivity PEDOT:PSS was first developed with a conductivity of 1 to , about three orders of magnitude lower than that of ITO ( ). While the electrical conductivity is not the most critical factor for a thin interlayer between ITO and organic active layer(s), the relatively high resistivity of PEDOT:PSS severely limits its application as a stand-alone electrode. Several methods have been successfully developed to improve the electrical conductivity of commercialized PEDOT:PSS. Some polar organic molecules with a high boiling temperature, such as dimethylsulfoxide (DMSO), 13 , 147 , 148 ethylene glycol (EG), 149 , 150 diethylene glycol, 151 and sorbitol, 17 , 152 have been mixed into the PEDOT:PSS aqueous solutions, which can enhance the film conductivity by more than one order of magnitude without affecting the transparency and high work function. For example, Na et al. reported highly conductive PEDOT:PSS layers with an average conductivity of by adding 5% DMSO to Baytron PH500 solution, compared to a film conductivity of without DMSO. 13 Using such a modified PEDOT:PSS layer to replace the bottom ITO electrode, the ITO-free P3HT:PCBM device showed an open-circuit voltage ( ) of 0.63 V, short-circuit current density ( ) of , fill factor (FF) of 53.5%, and of 3.27% under 1 sun simulated AM1.5 solar illumination, which was comparable with the ITO-based reference cell ( ) [see Fig. 7(a)]. The authors also explored the flexibility of the PEDOT:PSS films and its based device on PET substrates: as shown in Fig. 7(b), the resistance of the PEDOT:PSS film on a PET substrate remained nearly constant after 2500 bending cycles, while the resistance of conventional ITO films on PET substrate increased times after the bending, which led to a dramatic degradation in device efficiency. The ITO-based cell almost completely degraded after 75 cycles of bending, while the PEDOT:PSS-based devices showed nearly the same efficiency after 300 bending cycles. 13 Similarly, flexible OLEDs with PEDOT:PSS as the anodes have been reported by several research groups. 12 , 153 , 154 Wang et al. successfully demonstrated a flexible white OLED using a PEDOT:PSS anode with good device performance (power efficiency of at the brightness of was achieved) and mechanical flexibility (power efficiency reached after 100 bending cycles). 155 Some other techniques have also been demonstrated to further increase the PEDOT:PSS film conductivity. Kim et al. reported conductivities of for PEDOT:PSS films with the addition of 6 vol% EG, which were further increased to with solvent post-treatment (immerse the PEDOT:PSS films into EG and dry afterward). 37 Formic acid was also used to treat PEDOT:PSS for high conductivities: the highest conductivity of up to was successfully achieved with the formic acid treatment, approaching that of ITO. 156 According to these reports, the OPV cells based on the highly conductive PEDOT:PSS TEs exhibited the same efficiency as their ITO counterparts, which suggests their promising applications in optoelectronic devices. Fig. 7(a) characteristics of P3HT:PCBM devices with modified PH500 and ITO transparent electrodes. Insert: characteristics of P3HT:PCBM devices with four different anodes: VPAL4083, PH500, modified PH500, and ITO. (b) Relative resistance of ITO and PEDOT:PSS films on PET substrates as a function of number of bending cycles. Reprinted with permission from Ref. 13. Copyright 2008 John Wiley and Sons. 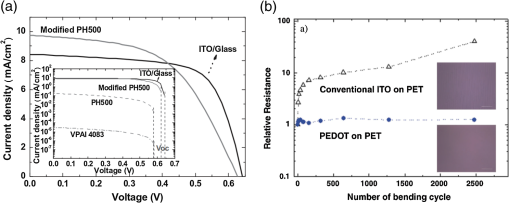 Furthermore, polymer electrodes can be utilized as the top TEs in OPV cells, either deposited directly onto the organic active materials using solution processing, such as spin coating, spray, or ink-jet printing, or indirectly using a stamp-transfer lamination process. 15 , 148 , 157 – 160 Gupta et al. applied a high conductivity PEDOT:PSS layer as a top TE in OPVs using a stamp-transfer method. Top-illuminated P3HT:PCBM devices were successfully fabricated on glass and opaque stainless steel substrates, which had maximum of 2.1 and 3.1% for normal and inverted polarity configurations, respectively. 15 For direct solution deposition, it is important to improve the wetting of the aqueous PEDOT:PSS solution on organic materials, which are typically hydrophobic. Several methods have been developed to solve this problem. 48 , 143 , 161 For example, Hau et al. demonstrated uniform PEDOT:PSS top electrodes on P3HT:PCBM active layers from diluted PEDOT:PSS solutions with isopropyl alcohol and n-butyl alcohol. 143 , 162 Some fluorosurfactants, such as Zonyl-FS300, are also used as additives to improve the wetting property of PEDOT:PSS solutions on organic films, which have negligible effects on the electrical and optical properties of PEDOT:PSS films but enables their deposition on most hydrophobic surfaces. 48 , 163 , 164 In summary, transparent polymers, especially the highly conductive PEDOT:PSS, are possible ITO substitutes for TEs, due to their high flexibility and excellent compatibility with large-area, solution-processed coating techniques. 12 , 165 , 166 The conductivity of PEDOT:PSS film is approaching that of ITO in certain cases, but further improvement may still be needed, especially when used in large-size, commercial scale devices. Other than doping, in situ polymerization of EDOT without adding in PSS components that significantly increase the film conductivity is one other possible method to reduce the PEDOT layer resistance. 152 , 167 – 170 Although there have been only a few reported examples on this topic, these attempts open up new routes to improve the electrical properties of polymer electrodes. Moreover, these polymers are known to degrade under humidity, high temperature, and UV exposure. 25 , 27 Last but not least, the environmental and operational stability of polymer TEs and their related devices must be improved before their extensive usage. 5.Nanomaterials as Transparent ElectrodesNanoscale materials have been widely studied in the past 20 years in terms of their controllable synthesis and attractive applications in many areas. Some nanomaterials are also being investigated as TEs, such as CNTs, graphene, and metal NWs. The attractive optoelectronic properties of these materials, combined with their high compatibility with solution-processed and large-scale manufacturing, make them very promising candidates as transparent conductive electrodes. 5.1.Carbon NanotubesSince the first report on CNTs in the early 1990s by Iijima, 171 CNTs have attracted extensive academic and industrial attention due to their unique and promising mechanical and electrical properties. Individual single-walled CNTs (SWCNTs) have charge mobilities of and electrical conductivities in the 1 to range. 10 , 172 Despite the excellent electrical properties of individual tubes, thin films with a network of CNTs show more than three orders of magnitude lower conductivity and mobility (the highest reported conductivity was and mobility was ) as a result of the large contact resistance between two CNTs. 10 Since the early 2000 s, there have been extensive discussions regarding the potential applications of CNTs as TEs in optoelectronic devices; 173 – 175 however, several impediments including CNT synthesis, purification, and film processes have hindered their commercial use. Solution processibility is one of the great advantages for nanoscale materials, which can be integrated with fast, large-scale, and high-throughput manufacturing methods. Therefore, dispersing the CNTs in compatible solvents or obtaining printable inks of CNTs is required for most solution-based deposition techniques. 176 Several studies have shown that unfunctionalized CNTs can be dispersed in some organic solvents, such as chloroform, dichlorobenzene, dimethylformamide, and cyclohexylpyrrolidone. However, the concentration of these CNT solutions has only reached 0.1 to , which limits their application for large-scale manufacturing. 176 – 179 Some dispersion agents or surfactants have been utilized to better promote the suspension of CNTs in organic solvents or even some aqueous media. 180 – 184 However, most surfactants in CNT solutions become impurities after forming solid films, leading to a significant decrease in film conductivity. 185 Some research groups have demonstrated that it is possible to achieve high concentration and well-dispersed CNT solutions by chemically modifying the CNT surface with covalently bonded functional groups. 176 , 186 Though such chemical modification assists the dispersion of CNTs and prevents the possible rebundling mechanisms, the anchored small molecules break up the ordered bonding structures and become defects in single CNTs, affecting the conductivity of films made from these solutions. Therefore, it is necessary to deeply understand the relationship between conductivity, surfactants, and molecular structures, which will provide guidance for achieving stable CNT solutions with controllable concentrations while maintaining the good electrical properties of single CNTs and CNT films. Several solution process techniques, including spin coating, 187 , 188 spray coating, 30 , 189 dip coating, 190 and solution-related soft lithography methods, 191 – 193 have been demonstrated to fabricate uniform transparent conductive CNT films. For example, Wu et al. reported a simple filtration technique to deposit uniform SWCNT films on various substrates. An SWCNT film was first vacuum-filtered on a filtration membrane from a dilute suspension of nanotubes, followed by purification processes to remove possible surfactants and impurities. The SWCNT film was then transferred onto any required substrates after dissolving the membrane in solvent. Benefiting from the vacuum filtration process, this method can provide homogeneous SWCNT films with precisely controllable thicknesses by varying the solution concentration and volume. 174 However, these deposition techniques are only effective for producing films on small area substrates and there are still many challenges to develop cheap and fast deposition techniques for large-scale and uniform CNT films. 194 , 195 Besides the processing challenges, the rough surfaces of CNT films combined with the high sheet resistance ( ) as a result of the possible defects and the poor interconnection between CNTs limit their applications in optoelectronic devices. In early reports, OPV devices with CNT films as TEs had of only , compared to 3 to 5% in standard ITO-based devices. 19 , 196 Many film treatment methods were developed for achieving CNT electrodes with higher electrical conductivity and smoother film surface. 20 , 26 , 30 , 197 , 198 Tenent et al. reported uniform SWCNT films by using an ultrasonic spray method [see the photograph in Fig. 8(a)] with the sheet resistance of , which was reduced to after nitric acid treatment. As shown in Fig. 8(b), an of 3.1% was achieved for a P3HT:PCBM device with the CNT electrode, which was only slightly lower than the ITO-based cells ( ). 189 Ou et al. demonstrated surface-modified nanotube films with a surface roughness and sheet resistance of using PEDOT:PSS modification, acid soaking, and polymer coating. With the modified CNT anodes, a maximum current efficiency of was achieved for an Alq3-based OLED, similar to the ITO-based reference devices. 199 Fig. 8(a) A photograph of single-walled carbon nanotube (SWNT) films ( ) by a spray deposition method. (b) characteristics of P3HT:PCBM devices with ITO and SWNT transparent electrodes. Reprinted with permission from Ref. 189. Copyright 2009 John Wiley and Sons. 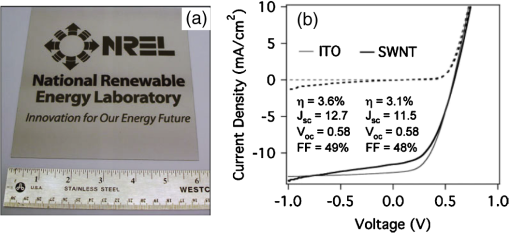 Although there are a few laboratory demonstrations using CNT networks as TEs in OPVs and OLEDs, it is still a long journey for their application in large-scale optoelectronic modules. First, achieving high-quality and low-cost CNTs over a large scale has been one of the challenges in the past 20 years, and will continue to be the research focus of this field in the future. 173 Though some scale-up technologies have been demonstrated by different research groups that have the potential for CNT mass productions, complicated purification processes, such as routes to remove remained impurities after synthesis and methods to distinguish the semiconducting and metallic components, still impede the scalability of high-quality CNTs and, hence, a reduction of the cost. 200 , 201 In terms of CNT thin films, the relatively low conductivity and transparency are still the two major factors that limit the device performance. The current deposition techniques for CNT films still need to be improved in their compatibility with large-scale roll-to-roll processing. Moreover, there is very limited study of the mechanical flexibility and environmental stability of devices with CNT electrodes. 5.2.GrapheneGraphene is a two-dimensional material of close-packed carbon atoms with a honeycomb crystal lattice, which can be considered as a single atomic layer of graphite. 202 Since the great breakthrough from its theoretical study to experimental studies in 2004, which led to the Nobel Prize in physics in 2010, 203 graphene has attracted tremendous attention and presented many possible applications in different areas. 204 , 205 Similar to CNTs where all carbon atoms are connected with -bonds, a single graphene sheet exhibits high in-plane conductance since all valence electrons are delocalized over the entire sheet. Moreover, graphene shows excellent optical properties: a single graphene layer has a theoretical transmittance of 97.7% (with a reflectance of and absorbance of ). 34 , 206 Besides the excellent optical transparency and electrical conductivity, graphene also exhibits other impressive properties, such as great thermal and chemical stability, excellent mechanical properties (high flexibility and stretchability), and good interfacial contact with organic materials, which enables its potential application as TE in optoelectronic devices. 14 , 207 – 210 Despite the promising properties of graphene as a TE, there are still several challenges remaining for its utilization in actual devices. Achieving high-quality graphene thin films and depositing graphene on required substrates is one of the biggest challenges. Since the pioneering report by Novoselov et al. in 2004, 211 mechanical exfoliation from bulk graphite materials is the basic technique to obtain high-quality graphene sheets for fundamental studies and small device demonstrations. 202 , 212 , 213 However, it is not suitable for high-throughput and large-scale industrial production lines. Chemical vapor deposition (CVD) is another successful technique for achieving graphene films, 32 , 47 , 214 – 216 which, despite its relatively high-cost deposition method with high treatment temperature, is still the most widely used fabrication method to obtain large graphene sheets. As shown in Figs. 9(a) and 9(b), Bae et al demonstrated large-scale (30-in.) CVD-grown graphene films with good flexibility and transparency. A monolayer of graphene film was first synthesized on copper foil using a CVD system, followed by some thermal and gas treatments for achieving high-quality films. A thermal-release tape was then attached with the graphene/copper foil for film transfer. After etching away the copper foil with copper etchant and cleaning the graphene surface with deionized water, the high-quality graphene film standing on the tape can be transferred to any target substrate. 215 Some solution-based deposition methods, such as liquid phase exfoliation and chemical reduction of graphene oxide, have also been developed to produce graphene films in the past several years. Direct exfoliation of graphite in specific solvents by sonication is the simple and low-cost method for graphene inks so far. 207 , 217 However, the concentration of graphene dispersed in these solvents, up to , is not high enough for some solution processes. Moreover, the mean flake size, generally on the order of 1 to , is relatively small due to the sonication; therefore, films prepared from these solutions exhibit higher resistance as a result of significant junction resistances. 218 This approach can be further improved by adding volatile agents (e.g., alkali salt) and organic surfactants into the original graphite dispersions, where the ions or organic molecules can intercalate between graphite layers and expand the interlayer spacing, leading to improved exfoliation and a more stabilized and better-dispersed graphene solution with a higher concentration (up to ). 219 – 221 Another alternative low-cost method is to reduce graphene oxide to graphene as demonstrated by several groups in the past few years, which provides a great opportunity to fabricate graphene inks or films on a large scale. 222 – 225 However, these solution methods, either exfoliation with metal ions and organic molecules or chemical reduction from oxides, induce a number of defects into the graphene sheets and interfaces, leading to a dramatic increase in the sheet resistance of the related graphene films. Fig. 9(a) and (b) Photographs of flexible graphene layers grown by a chemical vapor deposition technique. Reprinted with permission from Ref. 215. Copyright 2010 Nature Publishing Group. (c) Device structure of flexible phosphorescent OLED with a graphene electrode. (d) Current efficiency and luminance (insert) of phosphorescent OLEDs with ITO and graphene as transparent electrodes. Reprinted with permission from Ref. 47. Copyright 2012 Nature Publishing Group. 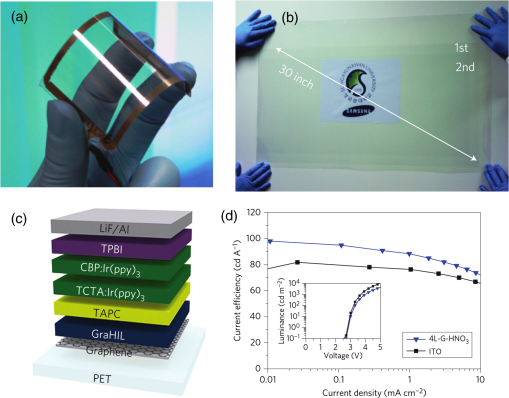 Although a single graphene sheet exhibits a low sheet resistance of , most graphene films produced by the above methods show a high sheet resistance on the order of a range as a result of the possible structural defects and contact resistances between graphene flakes. 14 , 207 , 222 De Arco et al. reported high-quality graphene films synthesized by CVD with a sheet resistance of and transmittance of 72% at . With such graphene films as TEs, a small molecule PV device with an of 1.18% was demonstrated, which was slightly lower than the ITO-reference cell ( ). 214 Some chemicals, such as , 31 , 32 , 215 and thionyl chloride, 226 have shown doping effects to improve the conductivity of graphene layers while maintaining the high levels of transmittance. For example, a CVD-grown, four-layer graphene film doped with was demonstrated with a sheet resistance as low as at transparency. 215 Han et al. used and as p-dopants in CVD-grown graphene films and applied similar highly conductive graphene films to replace ITO in flexible OLEDs, 47 resulting in a decrease in sheet resistance from 87 to 54 and , respectively. These resistance values are now approaching that of ITO and thin metal layers, and are much higher than that of PEDOT:PSS and CNT layers. Using such highly conductive graphene to replace ITO in a green phosphorescent OLED [see Figs. 9(c) and 9(d)], a very high current efficiency phosphorescent OLED of up to was achieved, which was appreciably higher than the ITO-reference cells ( ). 47 Apart from improving the conductivity of graphene films, interfacial modification between graphene electrodes and active layers is another challenge in efficient optoelectronic devices. Similar to other possible TE candidates, polymers, such as PEDOT:PSS, and metal oxides, such as , , and ZnO, are widely utilized to modify graphene interface and minimize the possible energy barriers for efficient injection/extraction of electrons and holes. In some cases, some interfacial layers are required to improve the surface wettability for solution-processed transport/active layers. For instance, Zhang et al. introduced a layer of Al nanoclusters on a single-layer graphene to overcome its hydrophobic properties for deposition. As a result of such surface modification, the P3HT:PCBM devices with graphene as TE showed an of , which was better than those without the modification and was close to that of the ITO-based references ( ). 227 These results suggest the great potential of using graphene as the TE in flexible organic optoelectronics. Moreover, graphene electrodes also possess outstanding mechanical flexibility. 14 , 216 De Arco et al. compared the graphene and ITO conductance under different bending conditions: only minor changes in the film morphology and conductance were observed for graphene films after bending the films to 160 deg, whereas ITO films exhibited irreversible cracks and a decrease in conductance under 60 deg of bending. Nevertheless, for large-area commercial applications, the resistance of the graphene layers needs to be further reduced, along with simplifying the graphene fabrication/transfer processes. Therefore, more effort is needed to obtain highly conductive graphene films with low-cost and large-scale deposition methods to enable its industrial application. 5.3.Metal NanowiresBesides the carbon-based nanomaterials, films with random networks of metal NWs are also attractive candidates for transparent conductive electrodes. 18 , 228 , 229 Although many metal nanostructures, such as copper, 230 , 231 gold, 232 , 233 and cupronickel, 234 have been demonstrated with promising properties and possibility as electrodes, Ag NWs have been the focus of research in this area due to the excellent electrical properties of bulk Ag materials and the synthesis scalability of Ag NWs. Ag NWs with a typical diameter of 20 to 40 nm and length of dispersed in water or organic solvents are now commercially available. Since metal NWs with proper surface treatment can be well dispersed in many solvents, many solution-based processing techniques, such as spin coating, 39 spray coating, 235 , 236 drop casting, 50 doctor blade coating, 11 , 237 Mayer rod coating, 40 brush painting, 238 etc., have been reported by different reach groups to achieve films with random NW networks. Similar to the discussion in CNTs, electrical and optical properties of the random NW networks are strongly dependent on the electrical properties of individual NWs, the interconnection between NWs, and the NW density in the resulting films. In general, the conductivity of NW networks increases as the length and diameter of an individual wire increases. 239 However, it is difficult to achieve NWs with an extremely long length and the typical reported length of Ag NWs is in the range of 1 to . It is also challenging to maintain the integrity of very long NWs in the solid-state film, as they tend to break during the dispersion and deposition processes. Controlling the diameter of metal NWs is also critical for achieving high-quality films. NWs in thin films provide the conductive pathways for charge carrier transport, but they also function as scatter centers to incident light affecting the optical properties of the films. Therefore, the diameter of metal NWs should be minimized in order to achieve high optical transparency. Moreover, the diameter of individual NWs determines the surface roughness of films with random NW networks, which should also be reduced for most optoelectronic devices. Furthermore, an optimized wire density is required to balance between the electrical and optical properties for specific applications. The junction resistance between the jointed wires is another dominant factor that affects the film resistance, which is similar to that for CNTs. 10 , 49 Several modification methods, such as thermal annealing, 35 , 228 optical sintering, 240 mechanical pressing, 241 and fusing with other materials, 42 have been demonstrated to reduce the junction resistance and improve the film conductivity. For example, Tokuno et al. applied a mechanical pressure of 25 MPa on as-deposited Ag NW films for 5 s at room temperature, which leads to a dramatic decrease in sheet resistance from to . 241 Several research groups have reported metal NW films with transmittance and sheet resistance [Figs. 10(a) and 10(b)], which are comparable with standard ITO electrodes, and explored them as TEs in organic optoelectronic devices. 11 , 39 , 242 Since the first application in small molecule OPVs that had an only , the overall performance of metal NW-based devices has been steadily advancing. Generally, a buffer layer (e.g., PEDOT:PSS, ZnO, or ) is required to flatten the surface of metal NW networks and to improve the contact with the active material so that they can serve as either anodes (collect/inject holes) or cathodes (collect/inject electrons) with proper buffer layers. 237 , 243 – 245 For instance, Leem et al. fabricated P3HT:PCBM cells with Ag NWs to replace the bottom ITO TE. With PEDOT:PSS and as buffer layers, normal and inverted device architectures were achieved, respectively, which had comparable of 2.0 and 3.5% compared to those of ITO-based reference cells. 39 Gaynor et al. successfully fabricated ITO-free white OLEDs with Ag NWs/poly (methyl methacrylate) composites as TEs, which showed a luminous efficiency and were close to that of the ITO-based devices ( ). 246 The solution-processibility of metal NWs also offers a great opportunity to make them as transparent top electrodes for organic optoelectronic devices. For example, Chen et al. successfully applied Ag NW electrodes in a high-performance polymer solar cell to achieve a visibly transparent device, where the ITO and ITO-nanoparticles-fused Ag NW layers serve as the bottom and top TEs, respectively. Such transparent devices showed efficiencies of and 3.8% when illuminated from ITO and Ag NW electrodes, respectively, and a maximum transmittance of in the visible light range. 247 Great mechanical properties are also the advantages of metal NW networks compared to ITO substrates. 40 , 88 , 248 , 249 As shown in Figs. 10(c) to 10(e), Yang et al. demonstrated the flexibility of Ag NW electrode based OPV devices on flexible substrates, which exhibited a recoverable efficiency under a bending angle of 120 deg. 40 Akter and Kim also studied the stretchability of Ag NW films, where the conductivity of the electrode remained unchanged with elongation. 248 Fig. 10(a) SEM image of a layer of Ag nanowires (NWs). (b) Transmittance and sheet resistance (insert) of Ag NW films with various thicknesses (different spin-speeds). Reprinted with permission from Ref. 39. Copyright 2011 John Wiley and Sons. (c) and (d) Experimental setup for measuring the flexibility of OPV devices with Ag NWs. (e) characteristics of a flexible OPV device under different bending conditions. Reprinted with permission from Ref. 40. Copyright 2011 American Chemical Society. 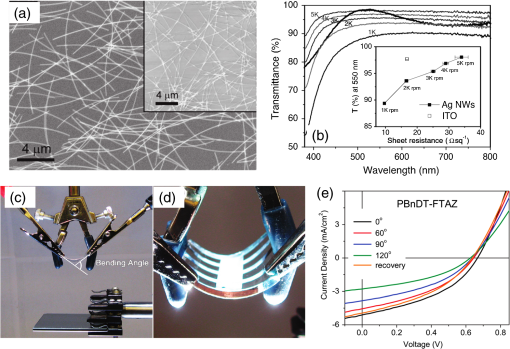 The above great device performance and excellent mechanical stability suggest the strong potential of using random metal NW networks as transparent conductive electrodes. However, several challenges still remain to be solved. For instance, the poor adhesion between the metal NWs and substrates limits their compatibility with different substrates. Adding some adhesive layers at the NW/substrate interface or treating metal NW surface with proper ligands to make them better anchored on substrates are some possible approaches to promote the adhesion. 228 The long-term stability of metal NW electrodes is another concern for their application in electronic devices. There has been some evidence suggesting that electromigration of metal atoms along the NWs causes failure of the wires and the entire network. This is especially important for devices that require high operation current, in which case the film conductivity keeps decreasing until their full breakdown. 250 , 251 Moreover, some metal NWs (e.g., Ag NWs) can be corroded through chemical reactions, such as oxidation and suffixation, which also cause the degradation of metal NWs and their related devices. 6.ConclusionTo date, ITO has been the most widely used material as transparent conductive electrodes in organic optoelectronic devices (OPVs and OLEDs). However, the rising cost of ITO, as a result of the scarcity of indium along with the increased demands, competes with the low-cost nature of organic-based devices. Therefore, searching for ITO alternatives becomes critical for the commercialization of low-cost OPVs and OLEDs. Various emerging materials have been reported as transparent conductive electrodes in optoelectronic devices, though not all of them show better properties than ITO. Thin metals are one of the alternatives to ITO, where their excellent ductility makes them suitable for flexible applications. However, the relatively low transparency of ultrathin metal layers limits the performance of their related devices. Conductive transparent polymers have emerged as low-cost electrodes over the last 10 years due to the great solution compatibility and large-scale processibility, but suffer from poor electrical properties and environmental stability. Nanomaterials, such as CNTs, graphenes, and metal NWs, have emerged as other attractive candidates as ITO alternatives. CNTs and graphenes have been studied extensively; however, they are still a long way from commercial applications as transparent conductive electrodes because of their low film conductivities and limited large-scale process techniques. Metal NWs, with their promising optoelectronic properties and good compatibility with most solution-based processes, are another top-rated material as transparent conductors, though several issues remain to be solved. The high surface roughness and poor electrical and environmental stability impede their applications in devices with ultrathin layers. Other than the focusing on one single material, the combination of two or more different materials, such as metal grid/polymer, metal NWs/polymer, graphene/metal grids, thin metal/graphene, etc., shows improved performance as transparent electrodes by complementing the good properties of each material. 252 , 253 This also open up more routes for searching TEs in optoelectronic devices. Overall, with cheap raw materials and good compatibility with environment-friendly deposition processes, we believe that these attractive materials will be adapted for application as TEs in optoelectronic devices. AcknowledgmentsThe authors gratefully acknowledge partial financial support from the Research Corporation for Science Advancement and the University of Florida Office of Research. References
S. R. Forrest
,
“The path to ubiquitous and low-cost organic electronic appliances on plastic,”
Nature, 428
(6986), 911
–918
(2004). http://dx.doi.org/10.1038/nature02498 0028-0836 Google Scholar
J. Xue
,
“Perspectives on organic photovoltaics,”
Polym. Rev., 50
(4), 411
–419
(2010). http://dx.doi.org/10.1080/15583724.2010.515766 0079-3736 Google Scholar
N. Espinosa
et al.
,
“Solar cells with one-day energy payback for the factories of the future,”
Energy Environ. Sci., 5
(1), 5117
–5132
(2012). http://dx.doi.org/10.1039/c1ee02728j 1754-5692 Google Scholar
W. Cao
J. Xue
,
“Recent progress in organic photovoltaics: device architecture and optical design,”
Energy Environ. Sci., 7 2123
–2144
(2014). http://dx.doi.org/10.1039/c4ee00260a 1754-5692 Google Scholar
G. Li
R. Zhu
Y. Yang
,
“Polymer solar cells,”
Nat. Photonics, 6
(3), 153
–161
(2012). http://dx.doi.org/10.1038/nphoton.2012.11 1749-4885 Google Scholar
M. A. Green
et al.
,
“Solar cell efficiency tables (version 43),”
Prog. Photovoltaics, 22
(1), 1
–9
(2014). http://dx.doi.org/10.1002/pip.v22.1 1062-7995 Google Scholar
C. W. Tang
,
“2-layer organic photovoltaic cell,”
Appl. Phys. Lett., 48
(2), 183
–185
(1986). http://dx.doi.org/10.1063/1.96937 0003-6951 Google Scholar
C. W. Tang
S. A. Vanslyke
,
“Organic electroluminescent diodes,”
Appl. Phys. Lett., 51
(12), 913
–915
(1987). http://dx.doi.org/10.1063/1.98799 0003-6951 Google Scholar
J. Xue
S. R. Forrest
,
“Carrier transport in multilayer organic photodetectors. II. Effects of anode preparation,”
J. Appl. Phys., 95
(4), 1869
–1877
(2004). http://dx.doi.org/10.1063/1.1640454 0021-8979 Google Scholar
D. S. Hecht
L. Hu
G. Irvin
,
“Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures,”
Adv. Mater., 23
(13), 1482
–1513
(2011). http://dx.doi.org/10.1002/adma.201003188 0935-9648 Google Scholar
J. Krantz
et al.
,
“Solution-processed metallic nanowire electrodes as indium tin oxide replacement for thin-film solar cells,”
Adv. Funct. Mater., 21
(24), 4784
–4787
(2011). http://dx.doi.org/10.1002/adfm.v21.24 1616-3028 Google Scholar
A. Sandstrom
et al.
,
“Ambient fabrication of flexible and large-area organic light-emitting devices using slot-die coating,”
Nat. Commun., 3 1002
(2012). http://dx.doi.org/10.1038/ncomms2002 2041-1723 Google Scholar
S.-I. Na
et al.
,
“Efficient and flexible ITO-free organic solar cells using highly conductive polymer anodes,”
Adv. Mater., 20
(21), 4061
–4067
(2008). http://dx.doi.org/10.1002/adma.v20:21 0935-9648 Google Scholar
K. S. Kim
et al.
,
“Large-scale pattern growth of graphene films for stretchable transparent electrodes,”
Nature, 457
(7230), 706
–710
(2009). http://dx.doi.org/10.1038/nature07719 0028-0836 Google Scholar
D. Gupta
M. M. Wienk
R. A. J. Janssen
,
“Efficient polymer solar cells on opaque substrates with a laminated PEDOT:PSS top electrode,”
Adv. Energy Mater., 3
(6), 782
–787
(2013). http://dx.doi.org/10.1002/aenm.201201061 1614-6832 Google Scholar
A. Kumar
C. Zhou
,
“The race to replace tin-doped indium oxide: which material will win?,”
ACS Nano, 4
(1), 11
–14
(2010). http://dx.doi.org/10.1021/nn901903b Google Scholar
F. Zhang
et al.
,
“Polymer photovoltaic cells with conducting polymer anodes,”
Adv. Mater., 14
(9), 662
–665
(2002). http://dx.doi.org/10.1002/(ISSN)1521-4095 0935-9648 Google Scholar
W. Gaynor
J.-Y. Lee
P. Peumans
,
“Fully solution-processed inverted polymer solar cells with laminated nanowire electrodes,”
ACS Nano, 4
(1), 30
–34
(2010). http://dx.doi.org/10.1021/nn900758e 1936-0851 Google Scholar
A. D. Pasquier
et al.
,
“Conducting and transparent single-wall carbon nanotube electrodes for polymer-fullerene solar cells,”
Appl. Phys. Lett., 87
(20), 203511
(2005). http://dx.doi.org/10.1063/1.2132065 0003-6951 Google Scholar
M. W. Rowell
et al.
,
“Organic solar cells with carbon nanotube network electrodes,”
Appl. Phys. Lett., 88
(23), 233506
(2006). http://dx.doi.org/10.1063/1.2209887 0003-6951 Google Scholar
V. Bhosle
et al.
,
“Gallium-doped zinc oxide films as transparent electrodes for organic solar cell applications,”
J. Appl. Phys., 102
(2), 023501
(2007). http://dx.doi.org/10.1063/1.2750410 0021-8979 Google Scholar
J. Y. Lee
et al.
,
“Solution-processed metal nanowire mesh transparent electrodes,”
Nano Lett., 8
(2), 689
–692
(2008). http://dx.doi.org/10.1021/nl073296g 1530-6984 Google Scholar
M.-G. Kang
et al.
,
“Organic solar cells using nanoimprinted transparent metal electrodes,”
Adv. Mater., 20
(23), 4408
–4413
(2008). http://dx.doi.org/10.1002/adma.v20:23 0935-9648 Google Scholar
T. Minami
,
“Substitution of transparent conducting oxide thin films for indium tin oxide transparent electrode applications,”
Thin Solid Films, 516
(7), 1314
–1321
(2008). http://dx.doi.org/10.1016/j.tsf.2007.03.082 0040-6090 Google Scholar
A. M. Nardes
et al.
,
“Conductivity, work function, and environmental stability of PEDOT: PSS thin films treated with sorbitol,”
Org. Electron., 9
(5), 727
–734
(2008). http://dx.doi.org/10.1016/j.orgel.2008.05.006 1566-1199 Google Scholar
Y. Feng
et al.
,
“Organic solar cells using few-walled carbon nanotubes electrode controlled by the balance between sheet resistance and the transparency,”
Appl. Phys. Lett., 94
(12), 123302
(2009). http://dx.doi.org/10.1063/1.3103557 0003-6951 Google Scholar
E. Vitoratos
et al.
,
“Thermal degradation mechanisms of PEDOT:PSS,”
Org. Electron., 10
(1), 61
–66
(2009). http://dx.doi.org/10.1016/j.orgel.2008.10.008 1566-1199 Google Scholar
M. Choe
et al.
,
“Efficient bulk-heterojunction photovoltaic cells with transparent multi-layer graphene electrodes,”
Org. Electron., 11
(11), 1864
–1869
(2010). http://dx.doi.org/10.1016/j.orgel.2010.08.018 1566-1199 Google Scholar
S. De
et al.
,
“Size effects and the problem with percolation in nanostructured transparent conductors,”
ACS Nano, 4
(12), 7064
–7072
(2010). http://dx.doi.org/10.1021/nn1025803 1936-0851 Google Scholar
S. Kim
et al.
,
“Spin- and spray-deposited single-walled carbon-nanotube electrodes for organic solar cells,”
Adv. Funct. Mater., 20
(14), 2310
–2316
(2010). http://dx.doi.org/10.1002/adfm.v20:14 1616-3028 Google Scholar
K. K. Kim
et al.
,
“Enhancing the conductivity of transparent graphene films via doping,”
Nanotechnology, 21
(28), 285205
(2010). http://dx.doi.org/10.1088/0957-4484/21/28/285205 0957-4484 Google Scholar
H. Park
et al.
,
“Doped graphene electrodes for organic solar cells,”
Nanotechnology, 21
(50), 505204
(2010). http://dx.doi.org/10.1088/0957-4484/21/50/505204 0957-4484 Google Scholar
H. Saarenpaa
et al.
,
“Aluminum doped zinc oxide films grown by atomic layer deposition for organic photovoltaic devices,”
Sol. Energy Mater. Sol. Cells, 94
(8), 1379
–1383
(2010). http://dx.doi.org/10.1016/j.solmat.2010.04.006 0927-0248 Google Scholar
J. Wu
et al.
,
“Organic light-emitting diodes on solution-processed graphene transparent electrodes,”
ACS Nano, 4
(1), 43
–48
(2010). http://dx.doi.org/10.1021/nn900728d 1936-0851 Google Scholar
X.-Y. Zeng
et al.
,
“A new transparent conductor: silver nanowire film buried at the surface of a transparent polymer,”
Adv. Mater., 22
(40), 4484
–4488
(2010). http://dx.doi.org/10.1002/adma.v22:40 0935-9648 Google Scholar
C.-K. Cho
et al.
,
“Mechanical flexibility of transparent PEDOT:PSS electrodes prepared by gravure printing for flexible organic solar cells,”
Sol. Energy Mater. Sol. Cells, 95
(12), 3269
–3275
(2011). http://dx.doi.org/10.1016/j.solmat.2011.07.009 0927-0248 Google Scholar
Y. H. Kim
et al.
,
“Highly conductive PEDOT:PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells,”
Adv. Funct. Mater., 21
(6), 1076
–1081
(2011). http://dx.doi.org/10.1002/adfm.201002290 1616-3028 Google Scholar
D. S. Hecht
R. B. Kaner
,
“Solution-processed transparent electrodes,”
MRS Bull., 36
(10), 749
–755
(2011). http://dx.doi.org/10.1557/mrs.2011.211 0883-7694 Google Scholar
D. S. Leem
et al.
,
“Efficient organic solar cells with solution-processed silver nanowire electrodes,”
Adv. Mater., 23
(38), 4371
–4375
(2011). http://dx.doi.org/10.1002/adma.201100871 0935-9648 Google Scholar
L. Yang
et al.
,
“Solution-processed flexible polymer solar cells with silver nanowire electrodes,”
ACS Appl. Mater. Interfaces, 3
(10), 4075
–4084
(2011). http://dx.doi.org/10.1021/am2009585 1944-8244 Google Scholar
S. D. Yambem
et al.
,
“Optimization of organic solar cells with thin film Au as anode,”
Sol. Energy Mater. Sol. Cells, 95
(8), 2424
–2430
(2011). http://dx.doi.org/10.1016/j.solmat.2011.04.019 0927-0248 Google Scholar
R. Zhu
et al.
,
“Fused silver nanowires with metal oxide nanoparticles and organic polymers for highly transparent conductors,”
ACS Nano, 5
(12), 9877
–9882
(2011). http://dx.doi.org/10.1021/nn203576v 1936-0851 Google Scholar
T. M. Barnes
et al.
,
“Comparing the fundamental physics and device performance of transparent, conductive nanostructured networks with conventional transparent conducting oxides,”
Adv. Energy Mater., 2
(3), 353
–360
(2012). http://dx.doi.org/10.1002/aenm.201100608 1614-6832 Google Scholar
W. Cao
et al.
,
“Flexible organic solar cells using an oxide/metal/oxide trilayer as transparent electrode,”
Org. Electron., 13
(11), 2221
–2228
(2012). http://dx.doi.org/10.1016/j.orgel.2012.05.047 Google Scholar
K. Ellmer
,
“Past achievements and future challenges in the development of optically transparent electrodes,”
Nat. Photonics, 6
(12), 808
–816
(2012). http://dx.doi.org/10.1038/nphoton.2012.282 1749-4885 Google Scholar
N. Formica
et al.
,
“Highly stable Ag-Ni based transparent electrodes on PET substrates for flexible organic solar cells,”
Sol. Energy Mater. Sol. Cells, 107 63
–68
(2012). http://dx.doi.org/10.1016/j.solmat.2012.08.002 0927-0248 Google Scholar
T.-H. Han
et al.
,
“Extremely efficient flexible organic light-emitting diodes with modified graphene anode,”
Nat. Photonics, 6
(2), 105
–110
(2012). http://dx.doi.org/10.1038/nphoton.2011.318 1749-4885 Google Scholar
M. Vosgueritchian
D. J. Lipomi
Z. Bao
,
“Highly conductive and transparent PEDOT:PSS films with a fluorosurfactant for stretchable and flexible transparent electrodes,”
Adv. Funct. Mater., 22
(2), 421
–428
(2012). http://dx.doi.org/10.1002/adfm.201101775 1616-3028 Google Scholar
D. Langley
et al.
,
“Flexible transparent conductive materials based on silver nanowire networks: a review,”
Nanotechnology, 24
(45), 452001
(2013). http://dx.doi.org/10.1088/0957-4484/24/45/452001 0957-4484 Google Scholar
M. Reinhard
et al.
,
“Solution-processed polymer-silver nanowire top electrodes for inverted semi-transparent solar cells,”
Org. Electron., 14
(1), 273
–277
(2013). http://dx.doi.org/10.1016/j.orgel.2012.10.039 1566-1199 Google Scholar
W. Zhang
et al.
,
“High-efficiency ITO-free polymer solar cells using highly conductive PEDOT:PSS/surfactant bilayer transparent anodes,”
Energy Environ. Sci., 6
(6), 1956
–1964
(2013). http://dx.doi.org/10.1039/c3ee41077c 1754-5692 Google Scholar
J.-S. Yu
et al.
,
“Transparent conductive film with printable embedded patterns for organic solar cells,”
Sol. Energy Mater. Sol. Cells, 109 142
–147
(2013). http://dx.doi.org/10.1016/j.solmat.2012.10.013 0927-0248 Google Scholar
D. Y. Cho
et al.
,
“Highly flexible and stretchable carbon nanotube network electrodes prepared by simple brush painting for cost-effective flexible organic solar cells,”
Carbon, 66 530
–538
(2014). http://dx.doi.org/10.1016/j.carbon.2013.09.035 0008-6223 Google Scholar
T. Minami
,
“Transparent conducting oxide semiconductors for transparent electrodes,”
Semicond. Sci. Techol., 20
(4), S35
–S44
(2005). http://dx.doi.org/10.1088/0268-1242/20/4/004 0268-1242 Google Scholar
I. Irfan
et al.
,
“Interplay of cleaning and de-doping in oxygen plasma treated high work function indium tin oxide (ITO),”
Org. Electron., 13
(10), 2028
–2034
(2012). http://dx.doi.org/10.1016/j.orgel.2012.05.036 1566-1199 Google Scholar
D. J. Milliron
et al.
,
“Surface oxidation activates indium tin oxide for hole injection,”
J. Appl. Phys., 87
(1), 572
–576
(2000). http://dx.doi.org/10.1063/1.371901 0021-8979 Google Scholar
C. N. Li
et al.
,
“Improved performance of OLEDs with ITO surface treatments,”
Thin Solid Films, 477
(1–2), 57
–62
(2005). http://dx.doi.org/10.1016/j.tsf.2004.08.111 0040-6090 Google Scholar
Y. Park
et al.
,
“Work function of indium tin oxide transparent conductor measured by photoelectron spectroscopy,”
Appl. Phys. Lett., 68
(19), 2699
–2701
(1996). http://dx.doi.org/10.1063/1.116313 0003-6951 Google Scholar
K. Sugiyama
et al.
,
“Dependence of indium-tin-oxide work function on surface cleaning method as studied by ultraviolet and x-ray photoemission spectroscopies,”
J. Appl. Phys., 87
(1), 295
–298
(2000). http://dx.doi.org/10.1063/1.371859 0021-8979 Google Scholar
K. Sun
J. Ouyang
,
“Polymer solar cells using chlorinated indium tin oxide electrodes with high work function as the anode,”
Sol. Energy Mater. Sol. Cells, 96
(1), 238
–243
(2012). http://dx.doi.org/10.1016/j.solmat.2011.10.002 0927-0248 Google Scholar
Z. R. Hong
et al.
,
“Characterization of organic photovoltaic devices with indium-tin-oxide anode treated by plasma in various gases,”
J. Appl. Phys., 100
(9), 093711
(2006). http://dx.doi.org/10.1063/1.2372574 0021-8979 Google Scholar
P. Destruel
et al.
,
“Influence of indium tin oxide treatment using UV-ozone and argon plasma on the photovoltaic parameters of devices based on organic discotic materials,”
Polym. Int., 55
(6), 601
–607
(2006). http://dx.doi.org/10.1002/(ISSN)1097-0126 1097-0126 Google Scholar
J. H. Huang
et al.
,
“Electrochemical characterization of the solvent-enhanced conductivity of poly(3,4-ethylenedioxythiophene) and its application in polymer solar cells,”
J. Mater. Chem., 19
(22), 3704
–3712
(2009). http://dx.doi.org/10.1039/b822729b 0959-9428 Google Scholar
B. Peng
et al.
,
“Performance improvement of polymer solar cells by using a solvent-treated poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) buffer layer,”
Appl. Phys. Lett., 98
(24), 243308
(2011). http://dx.doi.org/10.1063/1.3600665 0003-6951 Google Scholar
M. D. Irwin
et al.
,
“Structural and electrical functionality of NiO interfacial films in bulk heterojunction organic solar cells,”
Chem. Mater., 23
(8), 2218
–2226
(2011). http://dx.doi.org/10.1021/cm200229e 0897-4756 Google Scholar
S. R. Hammond
et al.
,
“Low-temperature, solution-processed molybdenum oxide hole-collection layer for organic photovoltaics,”
J. Mater. Chem., 22
(7), 3249
–3254
(2012). http://dx.doi.org/10.1039/c2jm14911g 0959-9428 Google Scholar
J. Meyer
et al.
,
“Transition metal oxides for organic electronics: energetics, device physics and applications,”
Adv. Mater., 24
(40), 5408
–5427
(2012). http://dx.doi.org/10.1002/adma.v24.40 0935-9648 Google Scholar
J. R. Manders
et al.
,
“Solution-processed nickel oxide hole transport layers in high efficiency polymer photovoltaic cells,”
Adv. Funct. Mater., 23
(23), 2993
–3001
(2013). http://dx.doi.org/10.1002/adfm.v23.23 1616-3028 Google Scholar
C. Waldauf
et al.
,
“Highly efficient inverted organic photovoltaics using solution based titanium oxide as electron selective contact,”
Appl. Phys. Lett., 89
(23), 233517
(2006). http://dx.doi.org/10.1063/1.2402890 0003-6951 Google Scholar
S. K. Hau
et al.
,
“Air-stable inverted flexible polymer solar cells using zinc oxide nanoparticles as an electron selective layer,”
Appl. Phys. Lett., 92
(25), 253301
(2008). http://dx.doi.org/10.1063/1.2945281 0003-6951 Google Scholar
L. Qian
et al.
,
“Hybrid polymer-CdSe solar cells with a ZnO nanoparticle buffer layer for improved efficiency and lifetime,”
J. Mater. Chem., 21
(11), 3814
–3817
(2011). http://dx.doi.org/10.1039/c0jm03799k 0959-9428 Google Scholar
Y. M. Sun
et al.
,
“Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer,”
Adv. Mater., 23
(14), 1679
–1683
(2011). http://dx.doi.org/10.1002/adma.201004301 0935-9648 Google Scholar
R. Zhou
et al.
,
“Solution-processed, nanostructured hybrid solar cells with broad spectral sensitivity and stability,”
Nanoscale, 4
(11), 3507
–3514
(2012). http://dx.doi.org/10.1039/c2nr30210a 1556-276X Google Scholar
P. K. H. Ho
et al.
,
“Molecular-scale interface engineering for polymer light-emitting diodes,”
Nature, 404
(6777), 481
–484
(2000). http://dx.doi.org/10.1038/35006610 0028-0836 Google Scholar
H. Yan
et al.
,
“High-brightness blue light-emitting polymer diodes via anode modification using a self-assembled monolayer,”
Adv. Mater., 15
(10), 835
–838
(2003). http://dx.doi.org/10.1002/adma.200304585 0935-9648 Google Scholar
S. Khodabakhsh
et al.
,
“Using self-assembling dipole molecules to improve hole injection in conjugated polymers,”
Adv. Funct. Mater., 14
(12), 1205
–1210
(2004). http://dx.doi.org/10.1002/(ISSN)1616-3028 1616-3028 Google Scholar
J. S. Kim
et al.
,
“Control of the electrode work function and active layer morphology via surface modification of indium tin oxide for high efficiency organic photovoltaics,”
Appl. Phys. Lett., 91
(11), 112111
(2007). http://dx.doi.org/10.1063/1.2778548 0003-6951 Google Scholar
M. G. Helander
et al.
,
“Chlorinated indium tin oxide electrodes with high work function for organic device compatibility,”
Science, 332
(6032), 944
–947
(2011). http://dx.doi.org/10.1126/science.1202992 0036-8075 Google Scholar
T. Dobbertin
et al.
,
“Inverted top-emitting organic light-emitting diodes using sputter-deposited anodes,”
Appl. Phys. Lett., 82
(2), 284
–286
(2003). http://dx.doi.org/10.1063/1.1535743 0003-6951 Google Scholar
H.-K. Kim
et al.
,
“Plasma damage-free sputtering of indium tin oxide cathode layers for top-emitting organic light-emitting diodes,”
Appl. Phys. Lett., 86
(18), 183503
(2005). http://dx.doi.org/10.1063/1.1923182 0003-6951 Google Scholar
G. Parthasarathy
et al.
,
“A metal-free cathode for organic semiconductor devices,”
Appl. Phys. Lett., 72
(17), 2138
–2140
(1998). http://dx.doi.org/10.1063/1.121301 0003-6951 Google Scholar
P. E. Burrows
et al.
,
“Semitransparent cathodes for organic light emitting devices,”
J. Appl. Phys., 87
(6), 3080
–3085
(2000). http://dx.doi.org/10.1063/1.372303 0021-8979 Google Scholar
H. Schmidt
et al.
,
“Efficient semitransparent inverted organic solar cells with indium tin oxide top electrode,”
Appl. Phys. Lett., 94
(24), 243302
(2009). http://dx.doi.org/10.1063/1.3154556 0003-6951 Google Scholar
G. Gu
et al.
,
“Transparent stacked organic light emitting devices. I. Design principles and transparent compound electrodes,”
J. Appl. Phys., 86
(8), 4067
–4075
(1999). http://dx.doi.org/10.1063/1.371331 0021-8979 Google Scholar
D. Kim
et al.
,
“Low temperature deposition of ITO thin films by ion beam sputtering,”
Thin Solid Films, 377 81
–86
(2000). http://dx.doi.org/10.1016/S0040-6090(00)01388-2 0040-6090 Google Scholar
Y. Hoshi
T. Kiyomura
,
“ITO thin films deposited at low temperatures using a kinetic energy controlled sputter-deposition technique,”
Thin Solid Films, 411
(1), 36
–41
(2002). http://dx.doi.org/10.1016/S0040-6090(02)00170-0 0040-6090 Google Scholar
P. E. Burrows
et al.
,
“Semitransparent cathodes for organic light emitting devices,”
J. Appl. Phys., 87
(6), 3080
–3085
(2000). http://dx.doi.org/10.1063/1.372303 0021-8979 Google Scholar
M. Song
et al.
,
“Highly efficient and bendable organic solar cells with solution-processed silver nanowire electrodes,”
Adv. Funct. Mater., 23
(34), 4177
–4184
(2013). http://dx.doi.org/10.1002/adfm.v23.34 1616-3028 Google Scholar
E. Fortunato
et al.
,
“Transparent conducting oxides for photovoltaics,”
MRS Bull., 32
(3), 242
–247
(2007). http://dx.doi.org/10.1557/mrs2007.29 0883-7694 Google Scholar
J. Meyer
et al.
,
“Indium-free transparent organic light emitting diodes with Al doped ZnO electrodes grown by atomic layer and pulsed laser deposition,”
Appl. Phys. Lett., 93
(7), 073308
(2008). http://dx.doi.org/10.1063/1.2975176 0003-6951 Google Scholar
H. Liu
et al.
,
“Efficient and ultraviolet durable inverted organic solar cells based on an aluminum-doped zinc oxide transparent cathode,”
Appl. Phys. Lett., 103
(4), 043309
(2013). http://dx.doi.org/10.1063/1.4816786 0003-6951 Google Scholar
J.-H. Park
et al.
,
“Effects of deposition temperature on characteristics of Ga-doped ZnO film prepared by highly efficient cylindrical rotating magnetron sputtering for organic solar cells,”
Sol. Energy Mater. Sol. Cells, 95
(2), 657
–663
(2011). http://dx.doi.org/10.1016/j.solmat.2010.09.036 0927-0248 Google Scholar
X. Jiang
et al.
,
“Aluminum-doped zinc oxide films as transparent conductive electrode for organic light-emitting devices,”
Appl. Phys. Lett., 83
(9), 1875
–1877
(2003). http://dx.doi.org/10.1063/1.1605805 0003-6951 Google Scholar
W. Wang
et al.
,
“Dependence of aluminum-doped zinc oxide work function on surface cleaning method as studied by ultraviolet and x-ray photoelectron spectroscopies,”
Appl. Surf. Sci., 257
(9), 3884
–3887
(2011). http://dx.doi.org/10.1016/j.apsusc.2010.11.084 0169-4332 Google Scholar
Z. Hu
et al.
,
“Highly efficient organic photovoltaic devices using F-doped SnO2 anodes,”
Appl. Phys. Lett., 98
(12), 123302
(2011). http://dx.doi.org/10.1063/1.3569758 0003-6951 Google Scholar
M. Liu
M. B. Johnston
H. J. Snaith
,
“Efficient planar heterojunction perovskite solar cells by vapour deposition,”
Nature, 501
(7467), 395
–398
(2013). http://dx.doi.org/10.1038/nature12509 0028-0836 Google Scholar
M. M. Lee
et al.
,
“Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites,”
Science, 338
(6107), 643
–647
(2012). http://dx.doi.org/10.1126/science.1228604 0036-8075 Google Scholar
J. Burschka
et al.
,
“Sequential deposition as a route to high-performance perovskite-sensitized solar cells,”
Nature, 499
(7458), 316
(2013). http://dx.doi.org/10.1038/nature12340 0028-0836 Google Scholar
S. H. Park
J. B. Park
P. K. Song
,
“Characteristics of Al-doped, Ga-doped and In-doped zinc-oxide films as transparent conducting electrodes in organic light-emitting diodes,”
Curr. Appl. Phys., 10
(3), S488
–S490
(2010). http://dx.doi.org/10.1016/j.cap.2010.02.036 1567-1739 Google Scholar
R. B. Pode
et al.
,
“Transparent conducting metal electrode for top emission organic light-emitting devices: Ca-Ag double layer,”
Appl. Phys. Lett., 84
(23), 4614
–4616
(2004). http://dx.doi.org/10.1063/1.1756674 0003-6951 Google Scholar
M. Al-Ibrahim
et al.
,
“Comparison of normal and inverse poly(3-hexylthiophene)/fullerene solar cell architectures,”
Sol. Energy Mater. Sol. Cells, 85
(2), 277
–283
(2005). http://dx.doi.org/10.1016/j.solmat.2004.08.001 0927-0248 Google Scholar
R. F. Bailey-Salzman
B. P. Rand
S. R. Forrest
,
“Semitransparent organic photovoltaic cells,”
Appl. Phys. Lett., 88
(23), 233502
(2006). http://dx.doi.org/10.1063/1.2209176 0003-6951 Google Scholar
B. O’Connor
et al.
,
“Transparent and conductive electrodes based on unpatterned, thin metal films,”
Appl. Phys. Lett., 93
(22), 223304
(2008). http://dx.doi.org/10.1063/1.3028046 0003-6951 Google Scholar
S. Wilken
et al.
,
“ITO-free inverted polymer/fullerene solar cells: interface effects and comparison of different semi-transparent front contacts,”
Sol. Energy Mater. Sol. Cells, 96
(1), 141
–147
(2012). http://dx.doi.org/10.1016/j.solmat.2011.09.044 0927-0248 Google Scholar
K.-S. Chen
et al.
,
“Semi-transparent polymer solar cells with 6% PCE, 25% average visible transmittance and a color rendering index close to 100 for power generating window applications,”
Energy Environ. Sci., 5
(11), 9551
–9557
(2012). http://dx.doi.org/10.1039/c2ee22623e 1754-5692 Google Scholar
S. D. Yambem
K. Liao
S. A. Curran
,
“Flexible Ag electrode for use in organic photovoltaics,”
Sol. Energy Mater. Sol. Cells, 95
(11), 3060
–3064
(2011). http://dx.doi.org/10.1016/j.solmat.2011.04.019 0927-0248 Google Scholar
S. Cheylan
et al.
,
“Organic light-emitting diode with indium-free metallic bilayer as transparent anode,”
Org. Electron., 12
(5), 818
–822
(2011). http://dx.doi.org/10.1016/j.orgel.2011.02.015 1566-1199 Google Scholar
E. H.-E. Wu
et al.
,
“Controlling optical properties of electrodes with stacked metallic thin films for polymeric light-emitting diodes and displays,”
J. Disp. Technol., 1
(1), 105
–111
(2005). http://dx.doi.org/10.1109/JDT.2005.853361 0193-2691 Google Scholar
H. M. Stec
et al.
,
“Ultrathin transparent Au electrodes for organic photovoltaics fabricated using a mixed mono-molecular nucleation layer,”
Adv. Funct. Mater., 21
(9), 1709
–1716
(2011). http://dx.doi.org/10.1002/adfm.201002021 1616-3028 Google Scholar
S. Schubert
et al.
,
“Improvement of transparent metal top electrodes for organic solar cells by introducing a high surface energy seed layer,”
Adv. Energy Mater., 3
(4), 438
–443
(2013). http://dx.doi.org/10.1002/aenm.v3.4 1614-6832 Google Scholar
C. J. Lee
et al.
,
“Red electrophosphorescent top emission organic light-emitting device with Ca/Ag semitransparent cathode,”
Appl. Phys. Lett., 89
(25), 253508
(2006). http://dx.doi.org/10.1063/1.2420770 0003-6951 Google Scholar
A. Haldar
et al.
,
“Organic photovoltaics using thin gold film as an alternative anode to indium tin oxide,”
Thin Solid Films, 519
(18), 6169
–6173
(2011). http://dx.doi.org/10.1016/j.tsf.2011.04.071 0040-6090 Google Scholar
J. C. C. Fan
et al.
,
“Transparent heat-mirror films of Tio2/Ag/Tio2 for solar-energy collection and radiation insulation,”
Appl. Phys. Lett., 25
(12), 693
–695
(1974). http://dx.doi.org/10.1063/1.1655364 0003-6951 Google Scholar
W. Ji
et al.
,
“High-color-rendering flexible top-emitting warm-white organic light emitting diode with a transparent multilayer cathode,”
Org. Electron., 12
(7), 1137
–1141
(2011). http://dx.doi.org/10.1016/j.orgel.2011.03.042 1566-1199 Google Scholar
H. Jin
et al.
,
“Efficient, large area ITO-and-PEDOT-free organic solar cell sub-modules,”
Adv. Mater., 24
(19), 2572
–2577
(2012). http://dx.doi.org/10.1002/adma.v24.19 0935-9648 Google Scholar
E. Wrzesniewski
et al.
,
“Enhancing light extraction in top-emitting organic light-emitting devices using molded transparent polymer microlens arrays,”
Small, 8
(17), 2647
–2651
(2012). http://dx.doi.org/10.1002/smll.v8.17 1613-6829 Google Scholar
M. Kohlstadt
et al.
,
“Inverted ITO- and PEDOT:PSS-free polymer solar cells with high power conversion efficiency,”
Sol. Energy Mater. Sol. Cells, 117 98
–102
(2013). http://dx.doi.org/10.1016/j.solmat.2013.05.023 0927-0248 Google Scholar
N. P. Sergeant
et al.
,
“Design of transparent anodes for resonant cavity enhanced light harvesting in organic solar cells,”
Adv. Mater., 24
(6), 728
–732
(2012). http://dx.doi.org/10.1002/adma.201104273 0935-9648 Google Scholar
K.-H. Choi
et al.
,
“Highly flexible and transparent InZnSnOx/Ag/InZnSnOx multilayer electrode for flexible organic light emitting diodes,”
Appl. Phys. Lett., 92
(22), 223302
(2008). http://dx.doi.org/10.1063/1.2937845 0003-6951 Google Scholar
E. Wrzesniewski
et al.
,
“Transparent oxide/metal/oxide trilayer electrode for use in top-emitting organic light-emitting diodes,”
J. Photon. Energy, 1 011023
(2011). http://dx.doi.org/10.1117/1.3592886 1947-7988 Google Scholar
L. Cattin
et al.
,
“Investigation of low resistance transparent MoO3/Ag/MoO3 multilayer and application as anode in organic solar cells,”
Thin Solid Films, 518
(16), 4560
–4563
(2010). http://dx.doi.org/10.1016/j.tsf.2009.12.031 0040-6090 Google Scholar
S.-H. Choa
et al.
,
“Mechanical integrity of flexible InZnO/Ag/InZnO multilayer electrodes grown by continuous roll-to-roll sputtering,”
Sol. Energy Mater. Sol. Cells, 95
(12), 3442
–3449
(2011). http://dx.doi.org/10.1016/j.solmat.2011.08.001 0927-0248 Google Scholar
H. Cho
et al.
,
“Highly flexible organic light-emitting diodes based on ZnS/Ag/WO3 multilayer transparent electrodes,”
Org. Electron., 10
(6), 1163
–1169
(2009). http://dx.doi.org/10.1016/j.orgel.2009.06.004 1566-1199 Google Scholar
K. Hong
et al.
,
“Optical properties of WO3/Ag/WO3 multi layer as transparent cathode in top-emitting organic light emitting diodes,”
J. Phys. Chem. C, 115
(8), 3453
–3459
(2011). http://dx.doi.org/10.1021/jp109943b 1932-7447 Google Scholar
H. Cho
C. Yun
S. Yoo
,
“Multilayer transparent electrode for organic light-emitting diodes: tuning its optical characteristics,”
Opt. Express, 18
(4), 3404
–3414
(2010). http://dx.doi.org/10.1364/OE.18.003404 1094-4087 Google Scholar
Y. C. Han
et al.
,
“ITO-free flexible organic light-emitting diode using ZnS/Ag/MoO3 anode incorporating a quasi-perfect Ag thin film,”
Org. Electron., 14
(12), 3437
–3443
(2013). http://dx.doi.org/10.1016/j.orgel.2013.09.014 1566-1199 Google Scholar
G. H. Jung
et al.
,
“BCP/Ag/MoO3 transparent cathodes for organic photovoltaics,”
Adv. Energy Mater., 1
(6), 1023
–1028
(2011). http://dx.doi.org/10.1002/aenm.201100411 1614-6832 Google Scholar
S. Lim
et al.
,
“Cu-based multilayer transparent electrodes: a low-cost alternative to ITO electrodes in organic solar cells,”
Sol. Energy Mater. Sol. Cells, 101 170
–175
(2012). http://dx.doi.org/10.1016/j.solmat.2012.01.016 0927-0248 Google Scholar
B. Tian
et al.
,
“Transparent organic light-emitting devices using a MoO3/Ag/MoO3 cathode,”
J. Appl. Phys., 110
(10), 104507
(2011). http://dx.doi.org/10.1063/1.3662194 0021-8979 Google Scholar
J.-A. Jeong
H.-K. Kim
,
“Low resistance and highly transparent ITO-Ag-ITO multilayer electrode using surface plasmon resonance of Ag layer for bulk-heterojunction organic solar cells,”
Sol. Energy Mater. Sol. Cells, 93
(10), 1801
–1809
(2009). http://dx.doi.org/10.1016/j.solmat.2009.06.014 0927-0248 Google Scholar
F. Roca
et al.
,
“Process development of amorphous silicon crystalline silicon solar cells,”
Sol. Energy Mater. Sol. Cells, 48
(1–4), 15
–24
(1997). http://dx.doi.org/10.1016/S0927-0248(97)00063-9 0927-0248 Google Scholar
U. P. Singh
S. P. Patra
,
“Progress in polycrystalline thin-film Cu(In,Ga)Se-2 solar cells,”
Int. J. Photoenergy, 2010 468147
(2010). http://dx.doi.org/10.1155/2010/468147 1110-662X Google Scholar
M.-G. Kang
et al.
,
“Transparent Cu nanowire mesh electrode on flexible substrates fabricated by transfer printing and its application in organic solar cells,”
Sol. Energy Mater. Sol. Cells, 94
(6), 1179
–1184
(2010). http://dx.doi.org/10.1016/j.solmat.2010.02.039 0927-0248 Google Scholar
C. Min
et al.
,
“Enhancement of optical absorption in thin-film organic solar cells through the excitation of plasmonic modes in metallic gratings,”
Appl. Phys. Lett., 96
(13), 133302
(2010). http://dx.doi.org/10.1063/1.3377791 0003-6951 Google Scholar
Y. Galagan
et al.
,
“ITO-free flexible organic solar cells with printed current collecting grids,”
Sol. Energy Mater. Sol. Cells, 95
(5), 1339
–1343
(2011). http://dx.doi.org/10.1016/j.solmat.2010.08.011 0927-0248 Google Scholar
J. Zou
et al.
,
“Metal grid/conducting polymer hybrid transparent electrode for inverted polymer solar cells,”
Appl. Phys. Lett., 96
(20), 203301
(2010). http://dx.doi.org/10.1063/1.3394679 0003-6951 Google Scholar
M.-G. Kang
et al.
,
“Efficiency enhancement of organic solar cells using transparent plasmonic Ag nanowire electrodes,”
Adv. Mater., 22
(39), 4378
–4383
(2010). http://dx.doi.org/10.1002/adma.v22:39 0935-9648 Google Scholar
Y.-H. Ho
et al.
,
“Transparent and conductive metallic electrodes fabricated by using nanosphere lithography,”
Org. Electron., 12
(6), 961
–965
(2011). http://dx.doi.org/10.1016/j.orgel.2011.03.019 1566-1199 Google Scholar
M.-G. Kang
L. J. Guo
,
“Nanoimprinted semitransparent metal electrodes and their application in organic light-emitting diodes,”
Adv. Mater., 19
(10), 1391
–1396
(2007). http://dx.doi.org/10.1002/(ISSN)1521-4095 0935-9648 Google Scholar
M.-G. Kang
L. J. Guo
,
“Semitransparent Cu electrode on a flexible substrate and its application in organic light emitting diodes,”
J. Vac. Sci. Technol. B, 25
(6), 2637
–2641
(2007). http://dx.doi.org/10.1116/1.2801873 0734-211X Google Scholar
K. Tvingstedt
O. Inganas
,
“Electrode grids for ITO-free organic photovoltaic devices,”
Adv. Mater., 19
(19), 2893
–2897
(2007). http://dx.doi.org/10.1002/(ISSN)1521-4095 0935-9648 Google Scholar
T. W. Lee
et al.
,
“Soft-contact optical lithography using transparent elastomeric stamps: application to nanopatterned organic light-emitting devices,”
Adv. Funct. Mater., 15
(9), 1435
–1439
(2005). http://dx.doi.org/10.1002/(ISSN)1616-3028 1616-3028 Google Scholar
S. K. Hau
et al.
,
“Indium tin oxide-free semi-transparent inverted polymer solar cells using conducting polymer as both bottom and top electrodes,”
Org. Electron., 10
(7), 1401
–1407
(2009). http://dx.doi.org/10.1016/j.orgel.2009.06.019 1566-1199 Google Scholar
Y. Zhou
et al.
,
“Indium tin oxide-free and metal-free semitransparent organic solar cells,”
Appl. Phys. Lett., 97
(15), 153304
(2010). http://dx.doi.org/10.1063/1.3499299 0003-6951 Google Scholar
Y.-S. Hsiao
et al.
,
“All-solution-processed inverted polymer solar cells on granular surface-nickelized polyimide,”
Org. Electron., 10
(4), 551
–561
(2009). http://dx.doi.org/10.1016/j.orgel.2009.01.012 1566-1199 Google Scholar
R. Po
et al.
,
“The role of buffer layers in polymer solar cells,”
Energy Environ. Sci., 4
(2), 285
–310
(2011). http://dx.doi.org/10.1039/c0ee00273a 1754-5692 Google Scholar
F. Nickel
et al.
,
“Cathodes comprising highly conductive poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) for semi-transparent polymer solar cells,”
Org. Electron., 11
(4), 535
–538
(2010). http://dx.doi.org/10.1016/j.orgel.2009.12.009 1566-1199 Google Scholar
Y. Zhou
et al.
,
“Optimization of a polymer top electrode for inverted semitransparent organic solar cells,”
Org. Electron., 12
(5), 827
–831
(2011). http://dx.doi.org/10.1016/j.orgel.2011.02.017 1566-1199 Google Scholar
B. Ouyang
et al.
,
“High-conductivity poly (3,4-ethylenedioxythiophene): poly(styrene sulfonate) film and its application in polymer optoelectronic devices,”
Adv. Funct. Mater., 15
(2), 203
–208
(2005). http://dx.doi.org/10.1002/(ISSN)1616-3028 1616-3028 Google Scholar
J.-R. Kim
et al.
,
“Efficient TCO-free organic solar cells with modified poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) anodes,”
J. Nanosci. Nanotechnol., 11
(1), 326
–330
(2011). http://dx.doi.org/10.1166/jnn.2011.3173 1533-4880 Google Scholar
Y.-S. Hsiao
et al.
,
“High-conductivity poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film for use in ITO-free polymer solar cells,”
J. Mater. Chem., 18
(48), 5948
–5955
(2008). http://dx.doi.org/10.1039/b813079e 0959-9428 Google Scholar
S. Admassie
et al.
,
“A polymer photodiode using vapour-phase polymerized PEDOT as an anode,”
Sol. Energy Mater. Sol. Cells, 90
(2), 133
–141
(2006). http://dx.doi.org/10.1016/j.solmat.2005.02.005 0927-0248 Google Scholar
P. A. Levermore
et al.
,
“High efficiency organic light-emitting diodes with PEDOT-based conducting polymer anodes,”
J. Mater. Chem., 18
(37), 4414
–4420
(2008). http://dx.doi.org/10.1039/b805994b 0959-9428 Google Scholar
G. Wang
X. Tao
R. Wang
,
“Flexible organic light-emitting diodes with a polymeric nanocomposite anode,”
Nanotechnology, 19
(14), 145201
(2008). http://dx.doi.org/10.1088/0957-4484/19/14/145201 0957-4484 Google Scholar
Y. Wang
et al.
,
“An efficient flexible white organic light-emitting device with a screen-printed conducting polymer anode,”
J. Phys. D Appl. Phys., 45
(40), 402002
(2012). http://dx.doi.org/10.1088/0022-3727/45/40/402002 0022-3727 Google Scholar
D. A. Mengistie
et al.
,
“Highly conductive PEDOT:PSS treated with formic acid for ITO-free polymer solar cells,”
ACS Appl. Mater. Interfaces, 6
(4), 2290
–2297
(2014). http://dx.doi.org/10.1021/am405024d 1944-8244 Google Scholar
T.-S. Kim
et al.
,
“All-solution-processed ITO-free polymer solar cells fabricated on copper sheets,”
Sol. Energy Mater. Sol. Cells, 98 168
–171
(2012). http://dx.doi.org/10.1016/j.solmat.2011.09.058 0927-0248 Google Scholar
D. J. Lipomi
et al.
,
“Electronic properties of transparent conductive films of PEDOT:PSS on stretchable substrates,”
Chem. Mater., 24
(2), 373
–382
(2012). http://dx.doi.org/10.1021/cm203216m 0897-4756 Google Scholar
Y.-C. Huang
et al.
,
“High-performance ITO-free spray-processed polymer solar cells with incorporating ink-jet printed grid,”
Org. Electron., 14
(11), 2809
–2817
(2013). http://dx.doi.org/10.1016/j.orgel.2013.08.001 1566-1199 Google Scholar
H. J. Lee
et al.
,
“Negative mold transfer patterned conductive polymer electrode for flexible organic light-emitting diodes,”
Org. Electron., 14
(1), 416
–422
(2013). http://dx.doi.org/10.1016/j.orgel.2012.11.015 1566-1199 Google Scholar
Q. Dong
et al.
,
“All-spin-coating vacuum-free processed semi-transparent inverted polymer solar cells with PEDOT:PSS anode and PAH-D interfacial layer,”
Org. Electron., 11
(7), 1327
–1331
(2010). http://dx.doi.org/10.1016/j.orgel.2010.04.012 1566-1199 Google Scholar
S. K. Hau
et al.
,
“Interfacial modification to improve inverted polymer solar cells,”
J. Mater. Chem., 18
(42), 5113
–5119
(2008). http://dx.doi.org/10.1039/b808004f 0959-9428 Google Scholar
Y. Zhou
et al.
,
“Investigation on polymer anode design for flexible polymer solar cells,”
Appl. Phys. Lett., 92
(23), 233308
(2008). http://dx.doi.org/10.1063/1.2945796 0003-6951 Google Scholar
M. M. Voigt
et al.
,
“Gravure printing for three subsequent solar cell layers of inverted structures on flexible substrates,”
Sol. Energy Mater. Sol. Cells, 95
(2), 731
–734
(2011). http://dx.doi.org/10.1016/j.solmat.2010.10.013 0927-0248 Google Scholar
T. Aernouts
et al.
,
“Printable anodes for flexible organic solar cell modules,”
Thin Solid Films, 451 22
–25
(2004). http://dx.doi.org/10.1016/j.tsf.2003.11.038 0040-6090 Google Scholar
F. C. Krebs
S. A. Gevorgyan
J. Alstrup
,
“A roll-to-roll process to flexible polymer solar cells: model studies, manufacture and operational stability studies,”
J. Mater. Chem., 19
(30), 5442
–5451
(2009). http://dx.doi.org/10.1039/b823001c 0959-9428 Google Scholar
Y. H. Ha
et al.
,
“Towards a transparent, highly conductive poly(3,4-ethylenedioxythiophene),”
Adv. Funct. Mater., 14
(6), 615
–622
(2004). http://dx.doi.org/10.1002/(ISSN)1616-3028 1616-3028 Google Scholar
J. P. Lock
S. G. Im
K. K. Gleason
,
“Oxidative chemical vapor deposition of electrically conducting poly(3,4-ethylenedioxythiophene) films,”
Macromolecules, 39
(16), 5326
–5329
(2006). http://dx.doi.org/10.1021/ma060113o 0024-9297 Google Scholar
Y. Chang
L. Wang
W. Su
,
“Polymer solar cells with poly(3,4-ethylenedioxythiophene) as transparent anode,”
Org. Electron., 9
(6), 968
–973
(2008). http://dx.doi.org/10.1016/j.orgel.2008.07.003 1566-1199 Google Scholar
M. V. Fabretto
et al.
,
“Polymeric material with metal-like conductivity for next generation organic electronic devices,”
Chem. Mater., 24
(20), 3998
–4003
(2012). http://dx.doi.org/10.1021/cm302899v 0897-4756 Google Scholar
S. Iijima
,
“Helical microtubules of graphitic carbon,”
Nature, 354
(6348), 56
–58
(1991). http://dx.doi.org/10.1038/354056a0 0028-0836 Google Scholar
B. S. Shim
et al.
,
“Integration of conductivity transparency, and mechanical strength into highly homogeneous layer-by-layer composites of single-walled carbon nanotubes for optoelectronics,”
Chem. Mater., 19
(23), 5467
–5474
(2007). http://dx.doi.org/10.1021/cm070442a 0897-4756 Google Scholar
R. H. Baughman
A. A. Zakhidov
W. A. de Heer
,
“Carbon nanotubes—the route toward applications,”
Science, 297
(5582), 787
–792
(2002). http://dx.doi.org/10.1126/science.1060928 0036-8075 Google Scholar
Z. Wu
et al.
,
“Transparent, conductive carbon nanotube films,”
Science, 305
(5688), 1273
–1276
(2004). http://dx.doi.org/10.1126/science.1101243 0036-8075 Google Scholar
M. Zhang
et al.
,
“Strong, transparent, multifunctional, carbon nanotube sheets,”
Science, 309
(5738), 1215
–1219
(2005). http://dx.doi.org/10.1126/science.1115311 0036-8075 Google Scholar
J. L. Bahr
et al.
,
“Dissolution of small diameter single-wall carbon nanotubes in organic solvents?,”
Chem. Commun., 193
–194
(2001). http://dx.doi.org/10.1039/b008042j 1364-548X Google Scholar
J. Chen
et al.
,
“Dissolution of full-length single-walled carbon nanotubes,”
J. Phys. Chem. B, 105
(13), 2525
–2528
(2001). http://dx.doi.org/10.1021/jp002596i 1520-6106 Google Scholar
H. T. Ham
Y. S. Choi
I. J. Chung
,
“An explanation of dispersion states of single-walled carbon nanotubes in solvents and aqueous surfactant solutions using solubility parameters,”
J. Colloid Interface Sci., 286
(1), 216
–223
(2005). http://dx.doi.org/10.1016/j.jcis.2005.01.002 0021-9797 Google Scholar
S. D. Bergin
et al.
,
“Towards solutions of single-walled carbon nanotubes in common solvents,”
Adv. Mater., 20
(10), 1876
–1881
(2008). http://dx.doi.org/10.1002/(ISSN)1521-4095 0935-9648 Google Scholar
R. Bandyopadhyaya
et al.
,
“Stabilization of individual carbon nanotubes in aqueous solutions,”
Nano Lett., 2
(1), 25
–28
(2002). http://dx.doi.org/10.1021/nl010065f 1530-6984 Google Scholar
M. Zheng
et al.
,
“DNA-assisted dispersion and separation of carbon nanotubes,”
Nat. Mater., 2
(5), 338
–342
(2003). http://dx.doi.org/10.1038/nmat877 1476-1122 Google Scholar
O. Matarredona
et al.
,
“Dispersion of single-walled carbon nanotubes in aqueous solutions of the anionic surfactant NaDDBS,”
J. Phys. Chem. B, 107
(48), 13357
–13367
(2003). http://dx.doi.org/10.1021/jp0365099 1520-6106 Google Scholar
D. S. Hecht
et al.
,
“Bioinspired detection of light using a porphyrin-sensitized single-wall nanotube field effect transistor,”
Nano Lett., 6
(9), 2031
–2036
(2006). http://dx.doi.org/10.1021/nl061231s 1530-6984 Google Scholar
R. Rastogi
et al.
,
“Comparative study of carbon nanotube dispersion using surfactants,”
J. Colloid Interface Sci., 328
(2), 421
–428
(2008). http://dx.doi.org/10.1016/j.jcis.2008.09.015 0021-9797 Google Scholar
H.-Z. Geng
et al.
,
“Absorption spectroscopy of surfactant-dispersed carbon nanotube film: modulation of electronic structures,”
Chem. Phys. Lett., 455
(4–6), 275
–278
(2008). http://dx.doi.org/10.1016/j.cplett.2008.02.102 0009-2614 Google Scholar
W. Huang
et al.
,
“Sonication-assisted functionalization and solubilization of carbon nanotubes,”
Nano Lett., 2
(3), 231
–234
(2002). http://dx.doi.org/10.1021/nl010083x 1530-6984 Google Scholar
T. P. Tyler
et al.
,
“Electronically monodisperse single-walled carbon nanotube thin films as transparent conducting anodes in organic photovoltaic devices,”
Adv. Energy Mater., 1
(5), 785
–791
(2011). http://dx.doi.org/10.1002/aenm.v1.5 1614-6832 Google Scholar
C. D. Williams
et al.
,
“Multiwalled carbon nanotube sheets as transparent electrodes in high brightness organic light-emitting diodes,”
Appl. Phys. Lett., 93
(18), 183506
(2008). http://dx.doi.org/10.1063/1.3006436 0003-6951 Google Scholar
R. C. Tenent
et al.
,
“Ultrasmooth, large-area, high-uniformity, conductive transparent single-walled-carbon-nanotube films for photovoltaics produced by ultrasonic spraying,”
Adv. Mater., 21
(31), 3210
–3216
(2009). http://dx.doi.org/10.1002/adma.v21:31 0935-9648 Google Scholar
Y. I. Song
et al.
,
“Flexible transparent conducting single-wall carbon nanotube film with network bridging method,”
J. Colloid Interface Sci., 318
(2), 365
–371
(2008). http://dx.doi.org/10.1016/j.jcis.2007.10.051 0021-9797 Google Scholar
J. Li
et al.
,
“Organic light-emitting diodes having carbon nanotube anodes,”
Nano Lett., 6
(11), 2472
–2477
(2006). http://dx.doi.org/10.1021/nl061616a 1530-6984 Google Scholar
D. Zhang
et al.
,
“Transparent, conductive, and flexible carbon nanotube films and their application in organic light-emitting diodes,”
Nano Lett., 6
(9), 1880
–1886
(2006). http://dx.doi.org/10.1021/nl0608543 1530-6984 Google Scholar
Y. H. Kim
et al.
,
“Semi-transparent small molecule organic solar cells with laminated free-standing carbon nanotube top electrodes,”
Sol. Energy Mater. Sol. Cells, 96
(1), 244
–250
(2012). http://dx.doi.org/10.1016/j.solmat.2011.10.001 0927-0248 Google Scholar
Y.-M. Chien
et al.
,
“A solution processed top emission OLED with transparent carbon nanotube electrodes,”
Nanotechnology, 21
(13), 134020
(2010). http://dx.doi.org/10.1088/0957-4484/21/13/134020 0957-4484 Google Scholar
L. Hu
et al.
,
“Flexible organic light-emitting diodes with transparent carbon nanotube electrodes: problems and solutions,”
Nanotechnology, 21
(15), 155202
(2010). http://dx.doi.org/10.1088/0957-4484/21/15/155202 0957-4484 Google Scholar
R. Ulbricht
et al.
,
“Transparent carbon nanotube sheets as 3-D charge collectors in organic solar cells,”
Sol. Energy Mater. Sol. Cells, 91
(5), 416
–419
(2007). http://dx.doi.org/10.1016/j.solmat.2006.10.002 0927-0248 Google Scholar
C. M. Aguirre
et al.
,
“Carbon nanotube sheets as electrodes in organic light-emitting diodes,”
Appl. Phys. Lett., 88
(18), 183104
(2006). http://dx.doi.org/10.1063/1.2199461 0003-6951 Google Scholar
Z. Yang
et al.
,
“Aligned carbon nanotube sheets for the electrodes of organic solar cells,”
Adv. Mater., 23
(45), 5436
–5439
(2011). http://dx.doi.org/10.1002/adma.201103509 0935-9648 Google Scholar
E. C.-W. Ou
et al.
,
“Surface-modified nanotube anodes for high performance organic light-emitting diode,”
ACS Nano, 3
(8), 2258
–2264
(2009). http://dx.doi.org/10.1021/nn900406n 1936-0851 Google Scholar
Q. Zhang
et al.
,
“Carbon nanotube mass production: principles and processes,”
Chemsuschem, 4
(7), 864
–889
(2011). http://dx.doi.org/10.1002/cssc.201100177 1864-5631 Google Scholar
M. F. L. De Volder
et al.
,
“Carbon nanotubes: present and future commercial applications,”
Science, 339
(6119), 535
–539
(2013). http://dx.doi.org/10.1126/science.1222453 0036-8075 Google Scholar
M. J. Allen
V. C. Tung
R. B. Kaner
,
“Honeycomb carbon: a review of graphene,”
Chem. Rev., 110
(1), 132
–145
(2010). http://dx.doi.org/10.1021/cr900070d 0009-2665 Google Scholar
M. S. Dresselhaus
P. T. Araujo
,
“Perspectives on the 2010 Nobel Prize in Physics for Graphene,”
ACS Nano, 4
(11), 6297
–6302
(2010). http://dx.doi.org/10.1021/nn1029789 Google Scholar
A. K. Geim
K. S. Novoselov
,
“The rise of graphene,”
Nat. Mater., 6
(3), 183
–191
(2007). http://dx.doi.org/10.1038/nmat1849 1476-1122 Google Scholar
F. Bonaccorso
et al.
,
“Graphene photonics and optoelectronics,”
Nat. Photonics, 4
(9), 611
–622
(2010). http://dx.doi.org/10.1038/nphoton.2010.186 1749-4885 Google Scholar
R. R. Nair
et al.
,
“Fine structure constant defines visual transparency of graphene,”
Science, 320
(5881), 1308
–1308
(2008). http://dx.doi.org/10.1126/science.1156965 0036-8075 Google Scholar
V. C. Tung
et al.
,
“High-throughput solution processing of large-scale graphene,”
Nat. Nanotechnol., 4
(1), 25
–29
(2009). http://dx.doi.org/10.1038/nnano.2008.329 1748-3387 Google Scholar
S. Pang
et al.
,
“Graphene as transparent electrode material for organic electronics,”
Adv. Mater., 23
(25), 2779
–2795
(2011). http://dx.doi.org/10.1002/adma.201100304 0935-9648 Google Scholar
M. He
et al.
,
“Graphene-based transparent flexible electrodes for polymer solar cells,”
J. Mater. Chem., 22
(46), 24254
–24264
(2012). http://dx.doi.org/10.1039/c2jm33784c 0959-9428 Google Scholar
J. Hwang
et al.
,
“Multilayered graphene anode for blue phosphorescent organic light emitting diodes,”
Appl. Phys. Lett., 100
(13), 133304
(2012). http://dx.doi.org/10.1063/1.3697639 0003-6951 Google Scholar
K. S. Novoselov
et al.
,
“Electric field effect in atomically thin carbon films,”
Science, 306
(5696), 666
–669
(2004). http://dx.doi.org/10.1126/science.1102896 0036-8075 Google Scholar
K. S. Novoselov
et al.
,
“Two-dimensional gas of massless Dirac fermions in graphene,”
Nature, 438
(7065), 197
–200
(2005). http://dx.doi.org/10.1038/nature04233 0028-0836 Google Scholar
Y. B. Zhang
et al.
,
“Fabrication and electric-field-dependent transport measurements of mesoscopic graphite devices,”
Appl. Phys. Lett., 86
(7), 073104
(2005). http://dx.doi.org/10.1063/1.1862334 0003-6951 Google Scholar
L. G. De Arco
et al.
,
“Continuous, highly flexible, and transparent graphene films by chemical vapor deposition for organic photovoltaics,”
ACS Nano, 4
(5), 2865
–2873
(2010). http://dx.doi.org/10.1021/nn901587x 1936-0851 Google Scholar
S. Bae
et al.
,
“Roll-to-roll production of 30-inch graphene films for transparent electrodes,”
Nat. Nanotechnol., 5
(8), 574
–578
(2010). http://dx.doi.org/10.1038/nnano.2010.132 1748-3387 Google Scholar
S. Lee
et al.
,
“Flexible organic solar cells composed of P3HT:PCBM using chemically doped graphene electrodes,”
Nanotechnology, 23
(34), 344013
(2012). http://dx.doi.org/10.1088/0957-4484/23/34/344013 0957-4484 Google Scholar
Y. Hernandez
et al.
,
“High-yield production of graphene by liquid-phase exfoliation of graphite,”
Nat. Nanotechnol., 3
(9), 563
–568
(2008). http://dx.doi.org/10.1038/nnano.2008.215 1748-3387 Google Scholar
U. Khan
et al.
,
“High-concentration solvent exfoliation of graphene,”
Small, 6
(7), 864
–871
(2010). http://dx.doi.org/10.1002/smll.v6:7 1613-6829 Google Scholar
L. M. Viculis
J. J. Mack
R. B. Kaner
,
“A chemical route to carbon nanoscrolls,”
Science, 299
(5611), 1361
–1361
(2003). http://dx.doi.org/10.1126/science.1078842 0036-8075 Google Scholar
C. Valles
et al.
,
“Solutions of negatively charged graphene sheets and ribbons,”
J. Am. Chem. Soc., 130
(47), 15802
(2008). http://dx.doi.org/10.1021/ja808001a 0002-7863 Google Scholar
H. X. Chang
et al.
,
“A transparent, flexible, low-temperature, and solution-processible graphene composite electrode,”
Adv. Funct. Mater., 20
(17), 2893
–2902
(2010). http://dx.doi.org/10.1002/adfm.201000900 1616-3028 Google Scholar
G. Eda
G. Fanchini
M. Chhowalla
,
“Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material,”
Nat. Nanotechnol., 3
(5), 270
–274
(2008). http://dx.doi.org/10.1038/nnano.2008.83 1748-3387 Google Scholar
D. Li
et al.
,
“Processable aqueous dispersions of graphene nanosheets,”
Nat. Nanotechnol., 3
(2), 101
–105
(2008). http://dx.doi.org/10.1038/nnano.2007.451 1748-3387 Google Scholar
H. A. Becerril
et al.
,
“Evaluation of solution-processed reduced graphene oxide films as transparent conductors,”
ACS Nano, 2
(3), 463
–470
(2008). http://dx.doi.org/10.1021/nn700375n 1936-0851 Google Scholar
Z. Yin
et al.
,
“Organic photovoltaic devices using highly flexible reduced graphene oxide films as transparent electrodes,”
ACS Nano, 4
(9), 5263
–5268
(2010). http://dx.doi.org/10.1021/nn1015874 1936-0851 Google Scholar
U. Dettlaff-Weglikowska
et al.
,
“Effect of SOCl2 treatment on electrical and mechanical properties of single-wall carbon nanotube networks,”
J. Am. Chem. Soc., 127
(14), 5125
–5131
(2005). http://dx.doi.org/10.1021/ja046685a 0002-7863 Google Scholar
D. Zhang
et al.
,
“Al-TiO2 composite-modified single-layer graphene as an efficient transparent cathode for organic solar cells,”
ACS Nano, 7
(2), 1740
–1747
(2013). http://dx.doi.org/10.1021/nn3058399 1936-0851 Google Scholar
S. De
et al.
,
“Silver nanowire networks as flexible, transparent, conducting films: extremely high DC to optical conductivity ratios,”
ACS Nano, 3
(7), 1767
–1774
(2009). http://dx.doi.org/10.1021/nn900348c 1936-0851 Google Scholar
H. Wu
et al.
,
“A transparent electrode based on a metal nanotrough network,”
Nat. Nanotechnol., 8
(6), 421
–425
(2013). http://dx.doi.org/10.1038/nnano.2013.84 1748-3387 Google Scholar
A. R. Rathmell
B. J. Wiley
,
“The synthesis and coating of long, thin copper nanowires to make flexible, transparent conducting films on plastic substrates,”
Adv. Mater., 23
(41), 4798
–4803
(2011). http://dx.doi.org/10.1002/adma.201102284 0935-9648 Google Scholar
D. Zhang
et al.
,
“Synthesis of ultralong copper nanowires for high-performance transparent electrodes,”
J. Am. Chem. Soc., 134
(35), 14283
–14286
(2012). http://dx.doi.org/10.1021/ja3050184 0002-7863 Google Scholar
T. L. Belenkova
et al.
,
“UV induced formation of transparent Au-Ag nanowire mesh film for repairable OLED devices,”
J. Mater. Chem., 22
(45), 24042
–24047
(2012). http://dx.doi.org/10.1039/c2jm35291e 0959-9428 Google Scholar
A. Sanchez-Iglesias
et al.
,
“Highly transparent and conductive films of densely aligned ultrathin Au nanowire monolayers,”
Nano Lett., 12
(12), 6066
–6070
(2012). http://dx.doi.org/10.1021/nl3021522 1530-6984 Google Scholar
A. R. Rathmell
et al.
,
“Synthesis of oxidation-resistant cupronickel nanowires for transparent conducting nanowire networks,”
Nano Lett., 12
(6), 3193
–3199
(2012). http://dx.doi.org/10.1021/nl301168r 1530-6984 Google Scholar
J. Krantz
et al.
,
“Spray-coated silver nanowires as top electrode layer in semitransparent P3HT:PCBM-based organic solar cell devices,”
Adv. Funct. Mater., 23
(13), 1711
–1717
(2013). http://dx.doi.org/10.1002/adfm.v23.13 1616-3028 Google Scholar
D. Y. Choi
et al.
,
“Annealing-free, flexible silver nanowire-polymer composite electrodes via a continuous two-step spray-coating method,”
Nanoscale, 5
(3), 977
–983
(2013). http://dx.doi.org/10.1039/c2nr32221h 1556-276X Google Scholar
T. Stubhan
et al.
,
“High fill factor polymer solar cells comprising a transparent, low temperature solution processed doped metal oxide/metal nanowire composite electrode,”
Sol. Energy Mater. Sol. Cells, 107 248
–251
(2012). http://dx.doi.org/10.1016/j.solmat.2012.06.039 0927-0248 Google Scholar
J.-H. Lee
et al.
,
“Brush painting of transparent PEDOT/Ag nanowire/PEDOT multilayer electrodes for flexible organic solar cells,”
Sol. Energy Mater. Sol. Cells, 114 15
–23
(2013). http://dx.doi.org/10.1016/j.solmat.2013.02.020 0927-0248 Google Scholar
S. M. Bergin
et al.
,
“The effect of nanowire length and diameter on the properties of transparent, conducting nanowire films,”
Nanoscale, 4
(6), 1996
–2004
(2012). http://dx.doi.org/10.1039/c2nr30126a 1556-276X Google Scholar
E. C. Garnett
et al.
,
“Self-limited plasmonic welding of silver nanowire junctions,”
Nat. Mater., 11
(3), 241
–249
(2012). http://dx.doi.org/10.1038/nmat3238 1476-1122 Google Scholar
T. Tokuno
et al.
,
“Fabrication of silver nanowire transparent electrodes at room temperature,”
Nano Res., 4
(12), 1215
–1222
(2011). http://dx.doi.org/10.1007/s12274-011-0172-3 1998-0124 Google Scholar
J.-Y. Lee
et al.
,
“Semitransparent organic photovoltaic cells with laminated top electrode,”
Nano Lett., 10
(4), 1276
–1279
(2010). http://dx.doi.org/10.1021/nl903892x 1530-6984 Google Scholar
W. Gaynor
et al.
,
“Smooth nanowire/polymer composite transparent electrodes,”
Adv. Mater., 23
(26), 2905
–2910
(2011). http://dx.doi.org/10.1002/adma.v23.26 0935-9648 Google Scholar
F. S. F. Morgenstern
et al.
,
“Ag-nanowire films coated with ZnO nanoparticles as a transparent electrode for solar cells,”
Appl. Phys. Lett., 99
(18), 183307
(2011). http://dx.doi.org/10.1063/1.3656973 0003-6951 Google Scholar
J. Ajuria
et al.
,
“Insights on the working principles of flexible and efficient ITO-free organic solar cells based on solution processed Ag nanowire electrodes,”
Sol. Energy Mater. Sol. Cells, 102 148
–152
(2012). http://dx.doi.org/10.1016/j.solmat.2012.03.009 0927-0248 Google Scholar
W. Gaynor
et al.
,
“Color in the corners: ITO-free white OLEDs with angular color stability,”
Adv. Mater., 25
(29), 4006
–4013
(2013). http://dx.doi.org/10.1002/adma.v25.29 0935-9648 Google Scholar
C.-C. Chen
et al.
,
“Visibly transparent polymer solar cells produced by solution processing,”
ACS Nano, 6
(8), 7185
–7190
(2012). http://dx.doi.org/10.1021/nn3029327 1936-0851 Google Scholar
T. Akter
W. S. Kim
,
“Reversibly stretchable transparent conductive coatings of spray-deposited silver nanowires,”
ACS Appl. Mater. Interfaces, 4
(4), 1855
–1859
(2012). http://dx.doi.org/10.1021/am300058j 1944-8244 Google Scholar
M. S. Miller
et al.
,
“Silver nanowire/optical adhesive coatings as transparent electrodes for flexible electronics,”
ACS Appl. Mater. Interfaces, 5
(20), 10165
–10172
(2013). http://dx.doi.org/10.1021/am402847y 1944-8244 Google Scholar
J. L. Elechiguerra
et al.
,
“Corrosion at the nanoscale: the case of silver nanowires and nanoparticles,”
Chem. Mater., 17
(24), 6042
–6052
(2005). http://dx.doi.org/10.1021/cm051532n 0897-4756 Google Scholar
J. Zhao
et al.
,
“Electrical breakdown of nanowires,”
Nano Lett., 11
(11), 4647
–4651
(2011). http://dx.doi.org/10.1021/nl202160c 1530-6984 Google Scholar
D. Zhang
et al.
,
“Polymer solar cells with gold nanoclusters decorated multi-layer graphene as transparent electrode,”
Appl. Phys. Lett., 99
(22), 223302
(2011). http://dx.doi.org/10.1063/1.3664120 0003-6951 Google Scholar
P. Lin
et al.
,
“Semitransparent organic solar cells with hybrid monolayer graphene/metal grid as top electrodes,”
Appl. Phys. Lett., 102
(11), 113303
(2013). http://dx.doi.org/10.1063/1.4798254 0003-6951 Google Scholar
BiographyWeiran Cao received his BS degree in materials science from University of Science and Technology of China in 2008 and his PhD degree in materials science and engineering from the University of Florida in 2013. He is currently a postdoctoral researcher in Professor Jiangeng Xue’s research group at the University of Florida. His current research work mainly focuses on novel optical designs for organic light-emitting devices and photovoltaic devices. Jian Li is an associate professor in the School of Materials at Arizona State University. He received his PhD in chemistry from the University of Southern California in 2005. His research interests include design and synthesis of advanced materials for the application in organic semiconductor devices, including organic light-emitting devices, organic photovoltaics, organic memory, and organic thin-film transistors. Hongzheng Chen is a professor in the Department of Polymer Science & Engineering at Zhejiang University. She received her PhD degree in polymer chemistry and physics from Zhejiang University in 1994. She visited Antwerpen University and Interuniversities MicroElectronic Center in Belgium during 1999 to 2001 and Stanford University in 2005 and 2007 as a visiting professor. Her research interests focus on organic (organic/inorganic) optoelectronic materials for photovoltaics, photodetection, transistors, and biosensors applications. Jiangeng Xue is a University of Florida Research Foundation professor and professor of materials science and engineering at the University of Florida. He was also an adjunct professor at Zhejiang University while on sabbatical leave in 2014. He received his PhD in electrical engineering from Princeton University in 2005. His research interests are broadly on the physics and processing of organic and organic-inorganic hybrid electronic materials and their applications in lighting, displays, photovoltaics, photodetection, and circuitry. |

