|
|
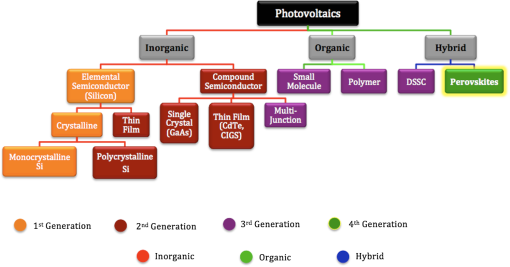
1.IntroductionSolar energy, an infinite producer of photons with a broad range of wavelengths, continues to be a potential source of clean energy. The photovoltaic (PV) process is considered as an ideal energy conversion process that can meet this requirement.1 The International Energy Agency’s technology roadmap estimates that by 2050, PV will provide of all global electricity production and avoid 2.3 gigatonnes of emissions per year.2 Given these predictions, photovoltaics or light-to-voltage converting devices have gained unprecedented attention from the research communities in the previous decades. Many researchers believe that solar cells, the basic building block of photovoltaics, are on the verge of creating a big impact by providing sustainable and efficient energy via cost-effective methods. The Sun’s energy can be harnessed in many ways. One example could be of a PV module that converts solar energy into electricity and a solar thermal collector that converts solar energy into heat, such as for domestic hot water or room heating.3 Hence, exploring ways to realize PV as cheaper, reliable, and durable ways to generate power is of interest to the scientific community. Silicon, the 14th element on the periodic table, is crucial for PV material in today’s world. However, there is still a need for newer materials and methodologies that offer better throughputs and efficiencies.4 The first generation of the solar cells dates back to 1953, when Gerald Pearson, Daryl Chapin, and Calvin Fuller discovered the silicon solar cell at AT&T Bell Labs.5 Currently, they trail on the efficiency chart at 25%.6 The use of polycrystalline silicon, thin films, and compound semiconductors rose in the era of second-generation solar cells, which entered the PV market in 1981.7 Currently, second-generation solar cells top the efficiency chart at 45%.8 In early 2000, organic solar cells paved way for a third wave of PV technology, which brings the advantages of flexibility, cost-effectiveness, and ease of fabrication. Though silicon-based solar cells continue to dominate over thin-film technologies and are flying high due to impressive efficiencies and better lifetimes, it is still in the interest of mankind to unwrap the organic horizon of solar cells. Therefore, there has been a greater demand in the past decade on scientists to discover flexible, cheaper, mass producible, and lightweight organic solar cells. One possible alternative to address this problem is to develop PV cells from materials that can be processed as easily as plastics. Augmenting this demand, the conferring of the Nobel Prize in Chemistry for the year 2000 to Dr. Alan Heeger in recognition of his work on conducting polymers has decisively paved a new era in the field of organic electronics, organic photovoltaics (OPVs), and organic/flexible displays.9 This has, in turn, led to the flexible third-generation solar cells, which are organic, dye-sensitized, and polymer. Figure 1 briefly differentiates inorganic solar cells from their organic counterparts. Organic solar cells (OSC) or plastic solar cells are an evolving multidisciplinary area of research that involve theoretical, experimental, and design challenges dealing with carbon-based materials and other organic compounds.10 It is a brand of polymer solar cell that incorporates a conductive organic polymer for light absorption, exciton dissociation, and charge transport to generate electricity.11 The OPVs based on a single conducting polymer can achieve efficiencies 12 and are still showing potential to increase this. They are different from the conventional silicon and other inorganic material based cells as they are cheaper and can be fabricated via low-cost solution processing techniques, such as spin coating, brush painting, and spray coating.4 These solution-processing techniques yield desired thicknesses of a few hundred nanometers and sober efficiencies of 4 to 5%. The wide multipolymer layered architectures of OSCs help execute the processes of photon trapping, generation of electrons and holes, and transport of charges to the respective cathodes and anodes. It was later reported that an effective way to improve polymer solar cell efficiency is to use a tandem structure; with this method, a broader part of the spectrum of solar radiation is used and the thermalization loss of photon energy is minimized. Yang Yang reported power conversion efficiencies of in tandem solar cells.13 Recently, Heliatek and Mitsubishi Chemicals have claimed the highest efficiency of 12 (Ref. 14) and 10%,15 respectively, for OSCs. Another kind of device is dye-sensitized solar cells (DSSCs), which have gained unparalleled growth in recent years due to their ease of fabrication and superior tunable optical properties. Recently, a DSSC with porphyrin sensitizers has achieved a record efficiency of 13% without sacrificing stability.16 The key drawbacks, such as efficiency, durability, and stability of OSCs and DSSCs, have made them the least chosen products for commercialization. In 2009, perovskites, a new solar cell material, evolved to transform photovoltaics and currently displays outstanding potential with power conversion efficiencies of 19.7%17 in the laboratory. Perovskite-based solar cells are purported to have the potential to provide sustainable and efficient power via cost-effective modes and techniques. Several research groups, such as Henry Snaith from Oxford University, Andrew Rappe at University of Pennsylvania, Sang II Seok at South Korean Institute KRICT, Michael Gratzel from EPFL, and Yang Yang from UCLA, are the frontrunners in the efforts to double the efficiency of these materials in less than a year.18–21 These devices are at the point of maximum optimism and are predicted to reach 50% efficiencies in the near future.22 These devices are also known for their high photon absorptivity, wide direct band gaps with superior carrier charge transports,23 and cost-effective modes of fabrication. 2.Significance of PerovskitesPerovskite is a mineral that came into existence when a German mineralogist, Gustav Rose, discovered calcium titanate () in 1839; it is named after a Russian mineralogist, Lew A. Perovski. Additionally, the compounds having a similar nomenclature to or the family of materials exhibiting the stoichiometry as are also known as perovskites. This ambiguity of terminology of the structural family and a mineral has been explicitly elucidated by Muller and Roy.24 They sought to address the ambiguity by proposing that the original mineral composition would be enclosed in square brackets. Thus, [] stands for the perovskite structures and not the composition . The A, B, and X in the perovskite crystal structure are typically represented as a larger rare earth metal cation, a smaller metal cation, and anions (, , , , or, in a few instances, ), respectively, arranged in octahedral symmetry as shown in Fig. 2. In the idealized (cubic) perovskite structure, the large A cations are in 12 coordinates and the smaller B cations occupy octahedral holes formed by the large X anions. There are many different perovskite materials, such as , , , , , , and the nonoxide , that, with skilled chemical manipulation, can produce an incredibly wide array of phases with a multitude of functionalities that include dielectric,25,26 ferroelectric,27–30 magnetoresistive,28 thermoelectric,31 electro-optic,32 semiconducting,33 conducting,28,34 and superconduction.29,35 Fig. 2(a) Typical octahedral structure of pervoskite crystal (). (b) Detailed features of perovskite solar devices. 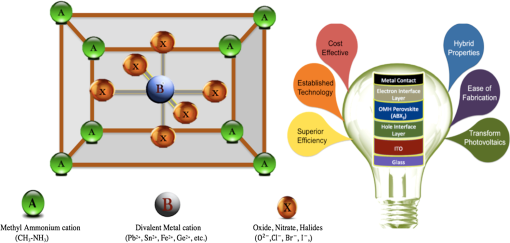 The concept of a two-dimensional layered organic-inorganic perovskite structure was derived from the three-dimensional (3-D) structure by cutting 3-D perovskite into one layer thick slice along the direction.36 It is known from the first-principles study that the replacement of an inorganic A cation in a basic cubic perovskite structure by a suitable organic cation provides a material of a superior scope with a broad selection of properties.37 In the early 1990s, an IBM researcher, David B. Mitzi, explored the structure-property relationship in organic-inorganic hybrid perovskite materials by replacing A with a cationic organic molecule, B with an inorganic post-transition metal, and X with halides, such as methylammonium tin iodide () and methyl ammonium lead iodide ().29 These organo-metal hallide (OMH) perovskite materials are engineered for diverse thin-film devices, such as solar cells,38,39 thin-film transistors,40 and light-emitting diodes.38,40 Strong diffraction peaks are observed at 14.02 deg and 28.3 deg, corresponding to the reflections from (110) and (220) crystal planes of the tetragonal perovskite structure on , which is shown in Fig. 3. Fig. 3X-ray diffraction peaks spectra of (a) on and (b) as-grown mesoporous . Reprinted with permission from Ref. 41. 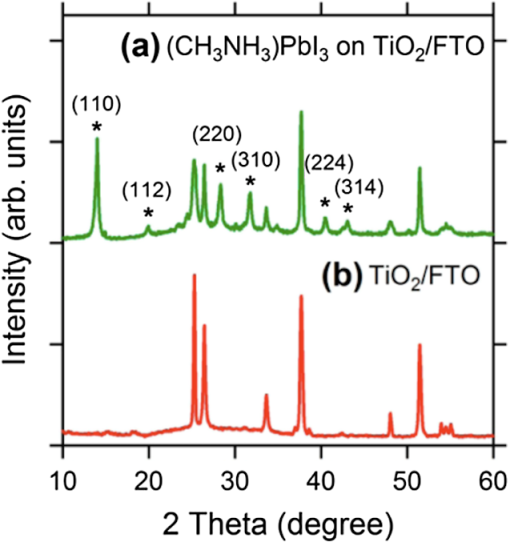 The nomenclatures with a methylammonium () organic cation and the inorganic metal halide octahedra’s (, ) create a blend of hybrid perovskites with evolving physical, optical, mechanical, and electrical properties. The OMH perovskites have strong intermolecular hydrogen bonds between the amino and halide group ions, whereas the weak Vander Waals exists among the organic ions. The divalent transition metal ions (such as , , , , , , , , etc.) function as the best metal cations for the organic-inorganic framework. Among these combinations, those belonging to group 14 (including and ) attracted more interest due to their good optoelectronic properties and potential for low-temperature device fabrication.20,34,42 The most predominantly used metal cations and with melting points of 505 and 600 K, respectively, are generally unreactive, stable at room temperature, and abundant in the Earth’s crust. The employment of the least electronegative halide anions improves the perovskite structures for strong absorption over wide band gaps. Additionally, lowering the Pauling electronegativity between the metal cation and halide anion can decrease the band gaps. The replacement of pure halides by mixed halides with changing ratios in the OMH perovskites can enhance the tuning capabilities of optical absorption or produce superior recombination properties.36 Therefore, the OMH structures such as are evolved. The geometrical size and structure of the organic cation is critical to make a best fit in the relatively small cuboctahedral hole, therefore, the A cation in the perovskite structure is limited to the smallest organic molecules, such as methylammonium ion (). However, this decrease in the A cation’s size has to be optimized by tightening the contact with anions in the cubic structure. This phenomenon of shrinking the geometrical constraints tends to distort the octahedral structure and introduces a distortion factor, also known as Goldschmidt’s tolerance factor.43 This is named after a Norwegian mineralogist, Viktor Moritz Goldschmidt, who studied a wide range of perovskite crystals and also helped to lay the foundation for the science of crystal chemistry. Typically, a Goldschmidt’s tolerance factor () of less than unity makes a suitable perovskite structure and is described as44 where , , and are the corresponding ionic radii of A, B, and X. For example, the larger organic methylammonium cation replaces A with an ionic radius of ();45 B with the most capable metal cation () and (); and the X anion with the uniquely distinguished halide ions (), (), and (). It is evident from the above geometries that the lead halide perovskites () have higher tolerations than their tin () counterparts; hence, the superior properties of lead over tin perovskites is demonstrated and the use of lead is justified.3.Atomistic Origin and Charge Transport Mechanism in OMH PerovskitesIn the diverse field of solar cells, the selection of materials of merit is critical and, in some cases, is presented as a trade-off factor among durability, stability, cost, ease of fabrication, and efficiency. An ideal solar cell material requires inheriting properties such as stronger and broader absorptions, ultrafast carrier charges separation and transport, high dielectric, and optimized diffusion lengths so that swift recombination occurs. By extending its organic and inorganic features to the bottlenecked efficiencies of organic PVs, the 3-D framework of methylammonium metal halide perovskite becomes an important modern scientific breakthrough. Typical absorption spectra of perovskite devices and its normalized spectra as a function of thickness are shown in Fig. 4. Fig. 4UV-Vis absorption spectra of (a) on and (b) normalized absoprtion as a function of thickness. Reprinted with permission from Ref. 41. 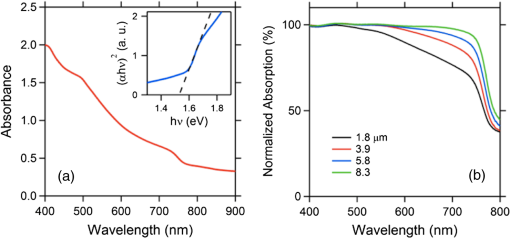 In 2009, Miyasaka et al. published a report on using OMH perovksites as visible light sensitizers yielding efficiencies of 3.81%, where it interacts with the conduction band levels of .39 Further investigations on these class of materials have revealed that they can augment the efficiencies and are exceptional to low electron and hole transport lengths, which are predominant among low-temperature solution-processed PV.21,46–48 3.1.Dielectric BehaviorThe compelling ferroelectric properties of 3-D structured OMH perovskites can be explained by the polar nature of methylammonium salt () with a permanent dipole moment, and the structural distortions carried by the lone pairs of lead () and tin (). These cubic superlattices consisting of semiconductor metal iodide layers sandwiched between insulator methylammonium layers can easily change their orientation and, thus, create the octahedral dielectric confinement of excitons.49 Typically, the excitons in organic molecules observe the Frenkel-Peierls model, leading to stronger bonds between them, higher exciton binding energies, and the least Bohr radius. These characteristics, consequently, tend to lower the dielectric constants and contribute to a poor charge transport in the conventional organic PV. On the contrary, the excitons in OMH perovskites exhibit the Wannier-Mott model30,50 due to their organic and inorganic behaviors. In these materials, the excitons experience the least binding energies and higher Bohr’s excitonic radius, thereby yielding superior charge transports. The exciton-evaluating components can be described by the well-known equations of binding energy () and the Bohr radius () of the excitons, where is the dielectric permittivity and is the exciton pair mass. Table 1 presents the trends in dielectric constants for various halides. However, the strong inherited polarization due to the ionic compounds (which have permanent dipole moment), the presence of an inorganic anion, and an organic cation yields higher dielectric constants, thus exhibiting a smooth mechanism for good long-range charge transport via band structure or polaron hopping.35 Table 1 details the dielectric and exciton behaviors in OMH perovskites.Table 1Dielectric and optical parameters of organo-metal halide (OMH) perovskites.
3.2.Charge TransportThe charge transport in OMH perovskite solar cells mostly resembles the conventional DSSCs. Zhao and Zhu report that the intensity-modulated photocurrent or photovoltage spectroscopies show that the transport and recombination properties of solid-state mesostructured perovskite solar cells are similar to those of solid-state DSSCs.41 They also report that the electron diffusion length decreases from 16.9 to as the film thickness increases from 1.8 to , revealing that the light absorption increases with an increasing film thickness, thereby allowing for faster recombination which limits the solar conversion process. Recently, Wehrenfennig et al. concluded that the OMH perovskites allow for an unexpected combination of both low charge recombination rates and high charge-carrier mobilities and are, therefore, the best candidates for light absorption and charge transport in solar cells.56 It is necessary to further explore the charge transports and electronic structures of OMH perovskite materials. The relativistic GW (Green’s function and Wick’s theorem of density functional theory) calculations on the electronic and optical properties predict to be a better electron transporter than .57 It is evident from Fig. 5 that the resistivity versus temperature of lead- and tin-based perovskites is similar to the characteristics of undoped semiconductor and displays a perfect ohmic behavior.33 Fig. 5The temperature-dependent resistivity plots of lead- and tin-based perovskite single crystals. Reprinted with permission from Ref. 33. 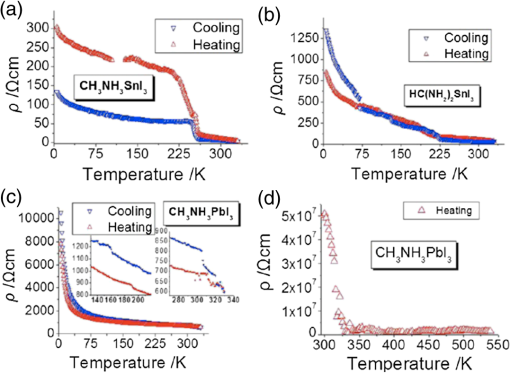 However, these OMH perovskite materials act as light sensitizers and ambipolar electron and hole transport materials,58 or they absorb light, thus creating the excitons (electron-hole pair). This involves steps like (1) creation of electron-hole pairs upon absorption of light by the perovskite; (2) formation of excitons after thermalization of the carriers; (3) charge separations at the junctions of electron and hole transport layers (HTLs); (4) injection of holes and electrons into the respective transport layers, such as spiro-2,2’,7,7’-tetrakis-(N,N-di-p-methoxyphenylamine)9,9’-spirobifluorene (OMeTAD) and ; (5) extraction of those charge carriers to the external circuit by contacts. Thus, it is vital to understand the OMH perovskite material stoichiometry and cell architecture to arrive at the best cell performance. So far, two types of device architectures, namely mesostructured and planar heterojunction, have been adapted. Figure 6 describes the band structure of a typical perovskite solar cell. The first mesostructure is derived from the conventional solid-state DSSCs, and in the latter, the perovskite layer is sandwiched between the electron (, , etc.) and hole [poly(3-hexylthiopene-2,5-diyl (P3HT), spiro-OMeTAD, etc.] transport layers. Figure 7(a) displays the typical cross-sectional SEM image of mesostructured architecture of perovskite solar cells and reveals that the pores of the mp- film are infiltrated with perovskite. The surface SEM image in Fig. 7(b) is crucial for determining the filling fraction and infiltration depth of and HTL into mp-. The transparent conducting oxide films, such as indium tin oxide (ITO) and fluorine-doped tin oxide (FTO), are widely used as electrodes due to their striking features such as low electrical resistance, high optical transmittance, and high photoconductivity.59 However, ITO and FTO are limited by the infrared wavelengths; in particular, the ITO-based substrates have low thermal stability, which explains the high efficiencies produced by FTOs. Fig. 6Energy band levels of showing the conduction and valence bands. Reprinted with permission from Ref. 48. 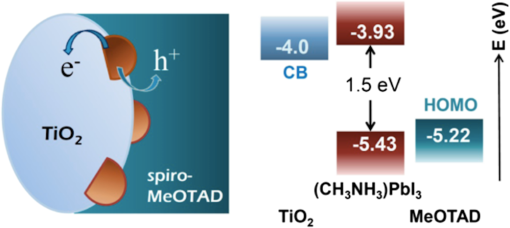 Fig. 7(a) Cross-sectional SEM image of organo-metal halide (OMH) perovskite. (b) Surface SEM image of on . Reprinted with permission from Ref. 60. 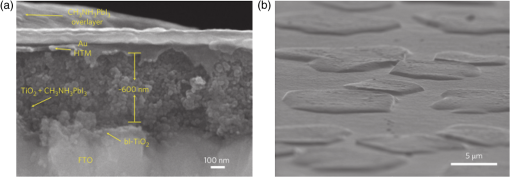 The mechanism of conduction depends largely on charge-carrier diffusion length, which generally means a thicker absorber layer for greater light trapping and is considered one of the key parameters of solar cell performance. After photoexcitation, the electron-hole pairs generated are dissociated in a few picoseconds (). The presence of metal oxide with a high electron affinity accelerates the formation of charges and motivates efficient electron injection for . The faster electron injection in is supported by the terahertz (THz) photoconductivity transient kinetic studies with normalized excitation density, which shows that the charge mobility of () is three to four times lower than that in neat and ().61 However, the unbalanced transport of charges resulting in space charge limited photocurrents and a lowering of the power conversion efficiency (PCE) can be attributed to the low intrinsic mobility of .62 A few other research groups have reported higher efficiencies of 15.7% with layers63 and 4.2% with buffer layer,58 respectively. The THz photoconductivity spectra of pristine OMH perovskite shown in Fig. 8 compares its composition with and . Slow electron–hole recombination and persistent high mobility are essential features for an efficient solar cell. The THz response along with other methods concluded that the THz mobilities of electrons are twice as mobile as the holes in the perovskite phase.61 Fig. 8Terahertz photoconductivity spectra of and at different pump probe delays. Reprinted with permission from Ref. 61. 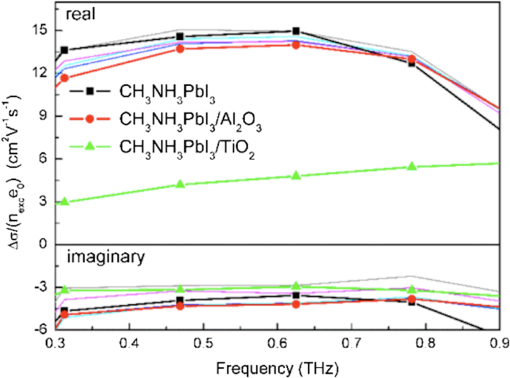 The degradation of OMH perovskites with humidity, ambient light, and oxidation can be attributed to the strong absorption onset of the material shifting from 1.6 to 2.4 eV. This low Urbach energy measured for perovskites can be a strong indicator that this material will not suffer from the Staebler–Wronski effect.64 The degradation of the cell performance is normally accompanied by a color bleaching of perovskites even when the cells are stored in the dark, suggesting that the chemical instability of perovskites in the iodide electrolyte contributes to the degradation of the cell performance over time. The exact degradation mechanism is still unknown, and it is crucial to know to what extent the material changes when measured at an ambient atmosphere or when exposed to liquid electrolytes. Therefore, a number of detailed degradation studies are required to further explore its usage for photoelectrochemical applications in the future. The OMH perovskite layer, typically with thicknesses of to 200 nm, determines the exciton diffusion lengths () and their lifetimes (), unlike the OSCs with a larger than these () materials (5 to 10 nm), and yields extended exciton lifetimes of (Ref. 65) when compared to 8 ns of OSC,66 motivating them to travel to the contacts before decay. Various research groups have investigated different halides (Cl, Br, and I),51,67,68 and mixed halides, such as , , or vice versa.38,50,63,69 However, the destructive effects of lead (Pb) brands tin (Sn) as a more suitable potential candidate for the perovskite structure. Mitzi investigated its () applicability for tuning the band gap, which was later introduced by various others31,33,34,36,38 as a key feature of conducting photovoltaics. A maximum PCE of 12.3% was achieved with . It is evident from Table 1 that the optical band gaps and exciton binding energies follow a trend, indicating the tunability and application-specific functionalities. However, a detailed study in different mixed halide materials is required to determine the electronic and optical properties. Typically, the perovskite solar cells suffer from high series and shunt resistance, thereby yielding poor fill factor values. This can be attributed to the higher conductivity of the perovskite layer being surpassed by the thicker layer of the lower conductive hole transport material (HTM) layer. Therefore, a thinner and better conductive HTM layer that enhances the hole mobility is highly desired for these devices. Bi et al. reported PCEs of 8.5, 4.5, and 1.6% by incorporating various HTMs, such as spiro-OMeTAD, P3HT, and 4-(diethylamino)-benzaldehyde diphenylhydrazone, respectively.70 Eventually, spiro-OMeTAD caught the attention of researchers and it was discovered that doping a lithium salt lithilum bis-trifluoromethanesulfonimide (Li-TFSI) can increase hole conductivity.71 The spectroscopy studies later revealed that the Fermi level in spiro-OMeTAD shifts toward the HOMO, and 24% of the spiro-OMeTAD molecules get oxidized in presence of Li-TFSI.72 Spiro-OMeTAD as a superior HTM layer is widely used to improve the device performance through enhancing its conductivity, as they mainly functioned to increase the hole mobility and charge density of HTMs, respectively, or increase both simultaneously.73 Figure 9 shows the J–V curves for various perovskite devices with mixed and pure compositions. Fig. 9J–V curves of various OMH perovskite devices: (a) , (b) , (c) , (d) graphene-, (e) , (f) . Reproduced with permission from Ref. 20. 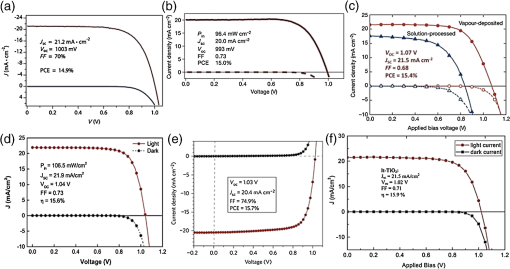 4.Fabrication ProceduresThe fabrication methodologies technically drive the performance and efficiency of perovskite solar cells, so it is vital to deposit the light sensitizer OMH perovskite material to enhance the device kinetics. The thinner cells tend to poorly absorb light, whereas in thicker cells, the charge carriers cannot travel through to reach the contacts.74 Despite their higher performance, they degrade at a faster rate, which is normally accompanied by a color bleaching due to the chemical instability of perovskites in the iodide electrolyte.41 Figure 10(a) showcases a multitude of techniques, such as solution processing, and deposition that can be demonstrated for the fabrication of perovskite-based solar cells. Fig. 10(a) Fabrication methods adopted for perovskite solar cells. (b) Architectures of typical perovskite solar cells. Reprinted with permission from Ref. 35. 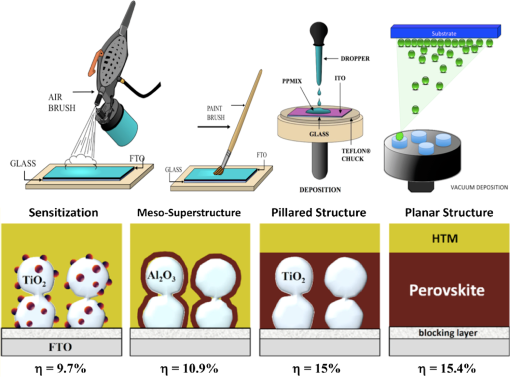 The ability to deposit these hybrid perovskites is very crucial to sustain their unique properties. So far, many researchers have determined the best fabrication procedures to be solution-processed techniques, evaporation, sequential deposition, and other techniques. Although the handiness of solution processing is widely encouraged, its drawbacks, such as poor wetting and inconsistency of hybrid materials, class them as a hindrance for an efficient solar device. An IBM researcher, Mitzi, has developed a novel melt processing technique for these hybrid films on flexible substrates.75 A few researchers crafted a unique low temperature processing technique that incorporates an scaffold in a perovskite material.76 Figure 10(b) illustrates various architectures investigated and efficiencies achieved for hybrid perovskite solar cells. A few researchers reported that increasing the conductivity of the HTMs by doping and optimizing charge collection by adjusting the absorber thickness could bring a positive impact on PCEs in planar heterojunction-based solar cells.73 The devices with longer diffusion lengths (1000 nm)77–79 are more efficient; therefore, the thicknesses and morphology of every functional layer is vital to drive the device to superior efficiencies. Edri et al. explained that the electrons could transport across only a short shallow barrier, whereas a hole can travel a long distance before recombining, thus clearly indicating that the makes an efficient solar cell (15%) with mesoporous scaffolds.54 Flexible devices on ITO/polyethylene terephthalate substrates can also be prepared by this route and display PCEs in excess of 10%. The combination of ease-of-fabrication, room-temperature processing, high device performance, and device flexibility are all expected to help enhance the most needed PV characteristics, such as efficient carrier collection and/or exciton dissociation, improved mobility within the electron transport layer, and extensive light scattering for these hybrid organic-inorganic solar devices. 5.Future OutlookThe usage of lead in perovskites deteriorates its culture for commercial applications, therefore, an exploratory drive to replace it with other divalent and least toxic metals, such as tin, copper, germanium, manganese, or iron, can change the dynamics of perovskite solar cells; current research is trending along these lines. The scientific community anticipates that these particular devices will make a successful entry to the commercial market by 2020. Further, exploration of superior materials that can tune ferroelectric domains and provide rapid support to the excitonic reactions in perovskite crystals can yield a better class of devices with the best durability and degradability. The recent advent of nanotechnology makes it worthwhile to investigate the role of nano-perovskites to enrich the performance and efficiency of any device. Additionally, the device cost can revolutionize the current photovoltaic technology, which is possible by developing more bench-top processes, such as printing, spraying, etc., rather than the clean room and high-tech equipment. Hence, there is a great need and urgency to develop an easy methodology of fabricating these devices. Some researchers believe that it can resemble the single crystal silicon and an OMH perovskite single crystal grown by melt-process or gel method will be eventually be a reality. Finally, its high transparency () is a predictor of superior functionalities by integrating these exotic devices in a tandem architecture. AcknowledgmentsThe authors acknowledge the DHS-BS and NSF-EPSCoR program and summer research program facilitated by Oak Ridge National Laboratory’s HBCU/MEI program. ReferencesS. Kazim et al.,
“Perovskite as light harvester: a game changer in photovoltaics,”
Angew. Chem. Int. Ed. Engl., 53
(11), 2812
–2824
(2014). http://dx.doi.org/10.1002/anie.v53.11 ACIEAY 0570-0833 Google Scholar
P. Frankl,
“Technology roadmap: solar photovoltaic energy,”
(2014) http://www.iea.org/publications/freepublications/publication/technology-roadmap-solar-photovoltaic-energy---foldout.html November ). 2014). Google Scholar
S. A. Kalogirou,
“Solar thermal collectors and applications,”
Prog. Energy Combust. Sci., 30
(3), 231
–295
(2004). http://dx.doi.org/10.1016/j.pecs.2004.02.001 PECSDO 0360-1285 Google Scholar
A. K. Chilvery et al.,
“A versatile technique for the fabrication of PEDOT: PSS films for organic solar cells,”
Energy, Sci. Technol., 4
(2), 6
–11
(2012). http://dx.doi.org/10.3968/j.est.1923847920120402.528 ESTEDA 0197-1417 Google Scholar
S.-S. Sun and N. S. Sariciftci, Organic Photovoltaics, 640 CRC Press, T&F Group, London
(2005). Google Scholar
“efficiency_chart,”
(2012). Google Scholar
M. A. Green,
“Crystalline and thin-film silicon solar cells: state of the art and future potential,”
Sol. Energy, 74
(3), 181
–192
(2003). http://dx.doi.org/10.1016/S0038-092X(03)00187-7 SRENA4 0038-092X Google Scholar
M. A. Green et al.,
“Solar cell efficiency tables,”
Prog. Photovolt. Res. Appl., 20 12
–20
(2012). http://dx.doi.org/10.1002/pip.v20.1 PPHOED 1062-7995 Google Scholar
H. Shirakawa et al.,
“Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH) x,”
J. Chem. Soc. Chem. Commun., 16 578
(1977). http://dx.doi.org/10.1039/c39770000578 JCCCAT 0022-4936 Google Scholar
M. Pagliaro, R. Ciriminna and G. Palmisano,
“Flexible solar cells,”
ChemSusChem, 1
(11), 880
–891
(2008). http://dx.doi.org/10.1002/cssc.v1:11 CHEMIZ 1864-5631 Google Scholar
A. C. Mayer et al.,
“Polymer-based solar cells,”
Mater. Today, 10
(11), 28
–33
(2007). http://dx.doi.org/10.1016/S1369-7021(07)70276-6 MATOBY 0096-4867 Google Scholar
G. Li, R. Zhu and Y. Yang,
“Polymer solar cells,”
Nat. Photonics, 6 153
–161
(2012). http://dx.doi.org/10.1038/nphoton.2012.11 1749-4885 Google Scholar
J. You et al.,
“A polymer tandem solar cell with 10.6% power conversion efficiency,”
Nat. Commun., 4 1446
(2013). http://dx.doi.org/10.1038/ncomms2411 NCAOBW 2041-1723 Google Scholar
Heliatek, “Heliatek press release,”
(2013) http://www.heliatek.com/?lang=en November 2014). Google Scholar
M. C. Scharber and N. S. Sariciftci,
“Efficiency of bulk-heterojunction organic solar cells,”
Prog. Polym. Sci., 38
(12), 1929
–1940
(2013). http://dx.doi.org/10.1016/j.progpolymsci.2013.05.001 PRPSB8 0079-6700 Google Scholar
P. Simon et al.,
“Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers,”
Nat. Chem., 6 242
–247
(2014). http://dx.doi.org/10.1038/nchem.1861 1755-4330 Google Scholar
H. Zhou et al.,
“Interface engineering of highly efficient perovskite solar cells,”
Science, 345
(6196), 542
–546
(2014). http://dx.doi.org/10.1126/science.1254050 SCIEAS 0036-8075 Google Scholar
M. Liu, M. B. Johnston and H. J. Snaith,
“Efficient planar heterojunction perovskite solar cells by vapour deposition,”
Nature, 501
(7467), 395
–398
(2013). http://dx.doi.org/10.1038/nature12509 NATUAS 0028-0836 Google Scholar
I. Grinberg et al.,
“Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials,”
Nature, 503
(7477), 509
–517
(2013). http://dx.doi.org/10.1038/nature12622 NATUAS 0028-0836 Google Scholar
P. Gao, M. Grätzel and M. K. Nazeeruddin,
“Organohalide lead perovskites for photovoltaic applications,”
Energy Environ. Sci., 7
(8), 2448
–2463
(2014). http://dx.doi.org/10.1039/c4ee00942h 1754-5692 Google Scholar
J. You et al.,
“Perovskite solar cells with high efficiency and flexibility,”
ACS Nano, 8
(2), 1674
–1680
(2014). 1936-0851 Google Scholar
B. Bulkin,
“Perovskites: the future of solar power?,”
Guardian, 1
(2014) http://www.theguardian.com/sustainable-business/perovskites-future-solar-power November ). 2014). Google Scholar
H. J. Snaith,
“Perovskites: the emergence of a new era for low-cost, high-efficiency solar cells,”
J. Phys. Chem. Lett., 4 3623
–3630
(2013). http://dx.doi.org/10.1021/jz4020162 1948-7185 Google Scholar
O. Muller and R. Roy,
“The major ternary structural families,”
in Cryst. Chem. Non-Metallic Mater.,
(1974). Google Scholar
E. J. Juarez-Perez et al.,
“Photoinduced giant dielectric constant in lead halide perovskite solar cells,”
J. Phys. Chem. Lett., 5
(13), 2390
–2394
(2014). http://dx.doi.org/10.1021/jz5011169 1948-7185 Google Scholar
A. K. Batra et al.,
“Simulation of energy harvesting from roads via pyroelectricity,”
J. Photonics Energy, 1
(1), 014001
(2011). http://dx.doi.org/10.1117/1.3656395 JPEOBV 1947-7988 Google Scholar
A. A. Bokov and Z. G. Ye,
“Recent progress in relaxor ferroelectrics with perovskite structure,”
J. Mater. Sci., 41
(1), 31
–52
(2006). http://dx.doi.org/10.1007/s10853-005-5915-7 JMTSAS 0022-2461 Google Scholar
M. A. Loi and J. C. Hummelen,
“Hybrid solar cells: perovskites under the Sun,”
Nat. Mater., 12
(12), 1087
–1089
(2013). http://dx.doi.org/10.1038/nmat3815 NMAACR 1476-1122 Google Scholar
D. B. Mitzi,
“Synthesis, structure, and properties of organic-inorganic perovskites and related materials,”
Progress in Inorganic Chemistry, 121 John Wiley & Sons, West Sussex, England, New York
(1999). Google Scholar
J. M. Frost et al.,
“Atomistic origins of high-performance in hybrid halide perovskite solar cells,”
Nano Lett., 14
(5), 2584
–2590
(2014). http://dx.doi.org/10.1021/nl500390f NALEFD 1530-6984 Google Scholar
Y. Takahashi et al.,
“Charge transport in tin-iodide perovskite: origin of high conductivity,”
Dalt. Trans., 40
(20), 5563
–5568
(2011). http://dx.doi.org/10.1039/c0dt01601b DTARAF 1477-9226 Google Scholar
A. Bhalla, R. Guo and R. Roy,
“The perovskite structure—a review of its role in ceramic science and technology,”
Mater. Res. Innov., 4
(1), 3
–26
(2000). http://dx.doi.org/10.1007/s100190000062 MRINFV 1432-8917 Google Scholar
C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis,
“Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties,”
Inorg. Chem., 52
(15), 9019
–9038
(2013). http://dx.doi.org/10.1021/ic401215x INOCAJ 0020-1669 Google Scholar
D. B. Mitzi et al.,
“Conducting tin halides with a layered organic-based perovskite structure,”
Nature, 369 467
–469
(1994). http://dx.doi.org/10.1038/369467a0 NATUAS 0028-0836 Google Scholar
H.-S. Kim, S. H. Im and N.-G. Park,
“Organolead halide perovskite: new horizons in solar cell research,”
J. Phys. Chem. C, 118
(11), 5615
–5625
(2014). http://dx.doi.org/10.1021/jp409025w 1932-7447 Google Scholar
J. L. Knutson, J. D. Martin and D. B. Mitzi,
“Tuning the band gap in hybrid tin iodide perovskite semiconductors using structural templating,”
Inorg. Chem., 44
(13), 4699
–4705
(2005). http://dx.doi.org/10.1021/ic050244q INOCAJ 0020-1669 Google Scholar
R. S. Roth,
“Classification of perovskite and other AB03 type compounds,”
J. Res. Natl. Bur. Stand. (1934)., 58
(2), 75
–88
(1957). http://dx.doi.org/10.6028/jres.058.010 JRNBAG 0160-1741 Google Scholar
D. B. Mitzi,
“Templating and structural engineering in organic-inorganic perovskites,”
J. Chem. Soc. Dalt. Trans., 1
(1), 1
–12
(2001). http://dx.doi.org/10.1039/b007070j JCSOA9 0368-1769 Google Scholar
A. Kojima et al.,
“Organometal halide perovskites as visible-light sensitizers for photovoltaic cells,”
J. Am. Chem. Soc., 131
(17), 6050
–6051
(2009). http://dx.doi.org/10.1021/ja809598r JACSAT 0002-7863 Google Scholar
D. B. Mitzi, K. Chondroudis and C. R. Kagan,
“Organic-inorganic electronics,”
IBM J. Res. Dev., 45
(1), 29
–45
(2001). http://dx.doi.org/10.1147/rd.451.0029 IBMJAE 0018-8646 Google Scholar
Y. Zhao and K. Zhu,
“Charge transport and recombination in perovskite () sensitized solar cells,”
J. Phys. Chem. Lett., 4 2880
–2884
(2013). http://dx.doi.org/10.1021/jz401527q 1948-7185 Google Scholar
I. B. Koutselas, L. Ducasse and G. C. Papavassiliou,
“Electronic properties of three- and low-dimensional semiconducting materials with Pb halide and Sn halide units,”
J. Phys. Condens. Matter, 8 1217
–1227
(1996). http://dx.doi.org/10.1088/0953-8984/8/9/012 JCOMEL 0953-8984 Google Scholar
X. W. Zhou, F. P. Doty and P. Yang,
“Atomistic models for scintillatory discovery,”
Proc. SPIE, 7806 78060E
(2010). http://dx.doi.org/10.1117/12.864152 PSISDG 0277-786X Google Scholar
V. M. Goldschmidt,
“Geochemisce Verterlungsgesetze der Elemente,”
Nor. Videnskap, Oslo, I kommission hos J. Dybwad, Oslo
(1927). Google Scholar
A. Poglitsch and D. Weber,
“Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimeter-wave spectroscopy,”
J. Chem. Phys., 87
(11), 6373
(1987). http://dx.doi.org/10.1063/1.453467 JCPSA6 0021-9606 Google Scholar
G. Xing et al.,
“Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3,”
Science, 342
(6156), 344
–347
(2013). http://dx.doi.org/10.1126/science.1243167 SCIEAS 0036-8075 Google Scholar
H. J. Snaith,
“Estimating the maximum attainable efficiency in dye-sensitized solar cells,”
Adv. Funct. Mater., 20
(1), 13
–19
(2010). http://dx.doi.org/10.1002/adfm.v20:1 AFMDC6 1616-3028 Google Scholar
N. Park,
“Organometal perovskite light absorbers toward a 20% efficiency low-cost solid-state mesoscopic solar cell,”
J. Phys. Chem. Lett., 4 2423
–2429
(2013). http://dx.doi.org/10.1021/jz400892a 1948-7185 Google Scholar
N. A. Gippius et al.,
“Dielectrically confined excitons and polaritons in natural superlattices—perovskite lead iodide semiconductors,”
J. Phys. IV, 3 437
–440
(1993). JPICEI 1155-4320 Google Scholar
S. a. Bretschneider et al.,
“Research update: physical and electrical characteristics of lead halide perovskites for solar cell applications,”
APL Mater., 2
(4), 040701
(2014). http://dx.doi.org/10.1063/1.4871795 AMPADS 2166-532X Google Scholar
H. Mashiyama and Y. Kurihara,
“Disordered cubic perovskite structure of (, Br, I),”
J. Korean Phys. Soc., 32 156
–158
(1998). KPSJAS 0374-4884 Google Scholar
Z. Chen et al.,
“Shape-controlled synthesis of organolead halide perovskite nanocrystals and their tunable optical absorption,”
Mater. Res. Express, 1
(1), 015034
(2014). http://dx.doi.org/10.1088/2053-1591/1/1/015034 2053-1591 Google Scholar
B. Conings et al.,
“Perovskite-based hybrid solar cells exceeding 10% efficiency with high reproducibility using a thin film sandwich approach,”
Adv. Mater., 26
(13), 2041
–2046
(2014). http://dx.doi.org/10.1002/adma.201304803 ADVMEW 0935-9648 Google Scholar
E. Edri et al.,
“High open-circuit voltage solar cells based on organic-inorganic lead bromide perovskite,”
J. Phys. Chem. Lett., 4
(6), 897
–902
(2013). http://dx.doi.org/10.1021/jz400348q 1948-7185 Google Scholar
N. Kitazawa, Y. Watanabe and Y. Nakamura,
“Optical properties of () and their mixed-halide crystals,”
J. Mater. Sci., 37 3585
–3587
(2002). http://dx.doi.org/10.1023/A:1016584519829 JMTSAS 0022-2461 Google Scholar
C. Wehrenfennig et al.,
“High charge carrier mobilities and lifetimes in organolead trihalide perovskites,”
Adv. Mater., 26
(10), 1584
–1589
(2014). http://dx.doi.org/10.1002/adma.v26.10 ADVMEW 0935-9648 Google Scholar
P. Umari, E. Mosconi and F. De Angelis,
“Relativistic GW calculations on and perovskites for solar cell applications,”
Sci. Rep., 4 4467
(2014). http://dx.doi.org/10.1038/srep04467 SRCEC3 2045-2322 Google Scholar
H.-S. Kim et al.,
“Mechanism of carrier accumulation in perovskite thin-absorber solar cells,”
Nat. Commun., 4 2242
(2013). http://dx.doi.org/10.1038/ncomms3242 NCAOBW 2041-1723 Google Scholar
D.-J. Kwak et al.,
“Comparison of transparent conductive indium tin oxide, titanium-doped indium oxide, and fluorine-doped tin oxide films for dye-sensitized solar cell application,”
J. Electr. Eng. Technol., 6
(5), 684
–687
(2011). http://dx.doi.org/10.5370/JEET.2011.6.5.684 1975-0102 Google Scholar
J. H. Heo et al.,
“Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors,”
Nat. Photonics, 7
(June), 486
–491
(2013). http://dx.doi.org/10.1038/NPHOTON.2013.80 1948-7185 Google Scholar
C. S. Ponseca et al.,
“Organometal halide perovskite solar cell materials rationalized: ultrafast charge generation, high and microsecond-long balanced mobilities, and slow recombination,”
J. Am. Chem. Soc., 136
(14), 5189
–5192
(2014). http://dx.doi.org/10.1021/ja412583t JACSAT 0002-7863 Google Scholar
E. Hendry et al.,
“Local field effects on electron transport in nanostructured revealed by terahertz spectroscopy,”
Nano Lett., 6 755
–759
(2006). http://dx.doi.org/10.1021/nl0600225 NALEFD 1530-6984 Google Scholar
M. M. Lee et al.,
“Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites,”
Science, 338
(6107), 643
–647
(2012). http://dx.doi.org/10.1126/science.1228604 SCIEAS 0036-8075 Google Scholar
S. De Wolf et al.,
“Organometallic halide perovskites: sharp optical absorption edge and its relation to photovoltaic performance,”
J. Phys. Chem. Lett., 5
(6), 1035
–1039
(2014). http://dx.doi.org/10.1021/jz500279b 1948-7185 Google Scholar
H.-S. Kim et al.,
“Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%,”
Sci. Rep., 2 591
(2012). http://dx.doi.org/10.1038/srep00591 SRCEC3 2045-2322 Google Scholar
R. L. Headrick et al.,
“Anisotropic mobility in large grain size solution processed organic semiconductor thin films,”
Appl. Phys. Lett., 92
(6), 063302
(2008). http://dx.doi.org/10.1063/1.2839394 APPLAB 0003-6951 Google Scholar
B. Suarez et al.,
“Recombination study of combined halides (Cl, Br, I) perovskite solar cells,”
J. Phys. Chem. Lett., 5
(10), 1628
–1635
(2014). http://dx.doi.org/10.1021/jz5006797 1948-7185 Google Scholar
M. Meister,
“Charge generation and recombination in hybrid organic / inorganic solar cells,”
250 Johannes Gutenberg-University Mainz,
(2013). Google Scholar
K. Liang, D. B. Mitzi and M. T. Prikas,
“Synthesis and characterization of organic—inorganic perovskite thin films prepared using a versatile two-step dipping technique,”
Chem. Mater., 10
(1), 403
–411
(1998). http://dx.doi.org/10.1021/cm970568f CMATEX 0897-4756 Google Scholar
D. Bi et al.,
“Using a two-step deposition technique to prepare perovskite () for thin film solar cells based on and mesostructures,”
RSC Adv., 3 18762
–18766
(2013). http://dx.doi.org/10.1039/c3ra43228a RSCACL 2046-2069 Google Scholar
H. Snaith and M. Gratzel,
“Enhanced charge mobility in a molecular hole transporter via addition of redox inactive ionic dopant: implication to dye-sensitized solar cells,”
Appl. Phys. Lett., 89 262114
(2006). http://dx.doi.org/10.1063/1.2424552 APPLAB 0003-6951 Google Scholar
R. Scholin et al.,
“Energy level shifts in spiro-OMeTAD molecular thin films when adding Li-TFSI,”
J. Phys. Chem., 116 26300
–26305
(2012). JPCHAX 0022-3654 Google Scholar
F. Liu et al.,
“Numerical simulation: toward the design of high-efficiency planar perovskite solar cells,”
Appl. Phys. Lett., 104
(25), 253508
(2014). http://dx.doi.org/10.1063/1.4885367 APPLAB 0003-6951 Google Scholar
G. Xing et al.,
“Long-range balanced electron- and hole-transport lengths in organic-inorganic ,”
Science, 342
(6156), 344
–347
(2013). http://dx.doi.org/10.1126/science.1243167 SCIEAS 0036-8075 Google Scholar
D. B. Mitzi, D. R. Medeiros and P. W. Dehaven,
“Low-temperature melt processing of organic-inorganic hybrid films,”
Chem. Mater., 14
(7), 2839
–2841
(2002). http://dx.doi.org/10.1021/cm020264f CMATEX 0897-4756 Google Scholar
M. Carnie et al.,
“A one-step low temperature processing route for organolead halide perovskite solar cells,”
Chem. Commun., 49 7893
–7895
(2013). http://dx.doi.org/10.1039/c3cc44177f CHCOFS 1364-548X Google Scholar
Y. Zhao, A. M. Nardes and K. Zhu,
“Solid-state mesostructured perovskite solar cells: charge transport, recombination, and diffusion length,”
J. Phys. Chem. Lett., 5 490
–494
(2014). http://dx.doi.org/10.1021/jz500003v 1948-7185 Google Scholar
V. Gonzalez-Pedro et al.,
“General working principles of perovskite solar cells,”
Nano Lett., 14
(2), 888
–893
(2014). http://dx.doi.org/10.1021/nl404252e NALEFD 1530-6984 Google Scholar
L. Barnea-nehoshtan et al.,
“Surface photovoltage spectroscopy study of organo-lead perovskite solar cells,”
Phys. Chem. Lett., 5 2408
–2413
(2014). http://dx.doi.org/10.1021/jz501163r 1948-7185 Google Scholar
|