|
|
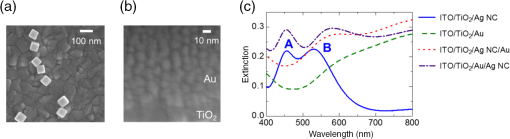
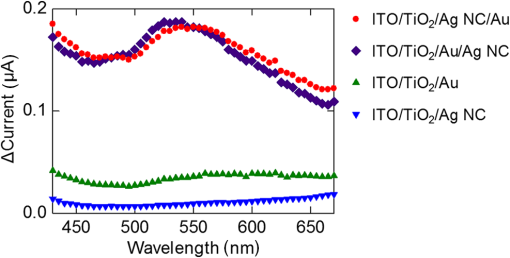
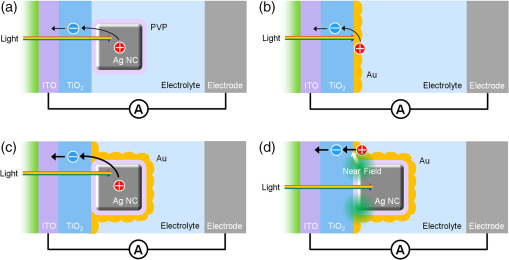
1.IntroductionPlasmonic light harvesting based on localized surface plasmon resonance (LSPR) of noble metal nanoparticles (NPs) has attracted much attention recently and is applied to photovoltaics and photocatalysis. There are two different ways of plasmonic light harvesting: (1) that based on a nanoantenna effect,1 in which a metal NP entraps a photon and transfers the obtained energy to a dye molecule or semiconductor via the optical near field generated in its vicinity, and (2) that based on plasmon-induced charge separation (PICS),2 in which an energetic electron is injected from a resonant NP to a semiconductor on contact. We reported PICS for the first time2 and indicated that it involves electron transfer from the plasmonic NPs to the semiconductor.2–7 The electron transfer involved is explained in terms of external photoelectric effect, hot electron injection, or interfacial electron transition, and recently some groups call it plasmonic hot electron (or carrier) injection. PICS has been widely applied to photovoltaics,2,8–12 photocatalysis,2,13,14 photochromism,15–17 photoactuation,18 and chemical sensing.19 A wide variety of plasmonic NPs have been used for PICS such as nanospheres,20 nanoplates,7 and halfshell arrays.21 NPs prepared by photocatalytic deposition2 or thermal dewetting of an evaporated film9 are suitable for PICS because of direct contact with the semiconductor, while chemically synthesized NPs may exhibit lower charge separation efficiency since they are covered with a protective agent. In the case of Au nanorods (NRs) protected with cetyltrimethylammonium bromide in contact with a thin film, we addressed the issue by photoelectrochemical deposition of Au from aqueous at both ends of the NRs under UV irradiation for excitation of .22 The Au NRs were thus converted to nanodumbbells (NDs), and NDs exhibited improved efficiency of PICS-based electron transfer to via the two spherical caps at both ends of NDs. Here, we propose an easier method to improve the PICS-based photocurrents. We use plasmonic Ag nanocubes (NCs) protected by poly(vinylpyrrolidone) (PVP) in this work. Although Ag NCs exhibit nanoantenna effects23–25 and various interesting optical properties,26–29 their PICS efficiency when they are in contact with is low because of the insulating PVP layer. In the present work, in order to improve the PICS efficiency, we coupled the Ag NCs simply with an evaporated Au thin film, which is coated on the Ag NCs placed on or coated directly on as the underlayer of Ag NCs. 2.Experiment2.1.Preparation of ElectrodesAg NCs were synthesized by the method reported by Im et al.30 with slight modifications28 [Fig. 1(a)]. The average edge length of the synthesized Ag NCs is about 80 nm. A transparent indium tin oxide (ITO) electrode was coated with a thin anatase film (60-nm thick, observed by scanning electron microscopy, SEM) by a spray pyrolysis method at 500°C from 2-propanol containing 0.028 M titanium diisopropoxide bis(acetylacetonate) as a precursor. The film was irradiated with UV light in order to make the surface sufficiently hydrophilic. The synthesized Ag NCs were drop-cast onto the surface (coverage ). If necessary, the substrate was coated with an Au film (ca. 5-nm thick, measured by quartz crystal microbalance) by vacuum evaporation before or after the drop-casting [Fig. 1(b)]. Thus, we prepared four different electrodes: NC, NC/Au, , and NC (). 2.2.Measurement of PhotocurrentsOne of the four electrodes was used as the working electrode. This electrode was connected to a potentiostat (SI 1280 B, Solartron) with an Ag|AgCl reference electrode and a Pt counter electrode and soaked in 0.1 M aqueous containing 0.5 M ethanol as an electron donor. The working electrode was polarized at versus Ag|AgCl and irradiated with monochromatic light (full width at , , and light ) using an optospectrum generator (Hamamatsu Photonics), and a steady-state photocurrent was measured at each wavelength. Virtually, the same photocurrents were observed even if the photoanode was short circuited to the counter electrode. 3.Results and Discussion3.1.Photocurrent Responses of the ITO / TiO2 / Ag NC ElectrodeAg NCs dispersed in ethanol exhibit the main extinction peak based on dipolar LSPR at 506 nm. When an Ag NC is adsorbed onto a substrate with a high refractive index, the LSPR peak splits into two peaks due to the distal and proximal modes.26 In the distal mode, electron oscillation is localized at the top of the NC away from the substrate, and in the proximal mode, the oscillation is localized at the bottom of the NC in contact with the substrate. The Ag NCs on show two peaks [Fig. 1(c)], namely peak A at 456 nm due to the distal mode and peak B at 530 nm due to the proximal mode. First, we measured photocurrent responses from the NC electrode irradiated from the backside with monochromatic light of different wavelengths in the electrolyte solution. Three independently prepared electrodes were examined and the average currents were plotted against the irradiation wavelength (Fig. 2). Small anodic photocurrents were observed in the wavelength range examined (430 to 670 nm). Conduction electrons in an Ag NC oscillate in resonance with electric field oscillation of the incident light, and an electron is injected from the resonant NC to the conduction band of , resulting in PICS.2 As a result, the anodic photocurrent flows [Fig. 3(a)]. However, the photocurrents were very low and no obvious current peaks corresponding to the distal and proximal modes were obtained. The low currents could be explained in terms of a blocking effect of insulating PVP, which covers and protects the NC surface. Capping agents like PVP are known to block access of reactant molecules.31,32 Likewise, the PVP layer should also inhibit the electron transfer from the Ag NC to . 3.2.Effect of a Thin Gold CoatingWe therefore coated the Ag NCs on with a thin Au layer (ca. 5 nm thick) by evaporation, envisaging promotion of the electron transfer. The photocurrent responses from the NC/Au electrode thus prepared were measured and are shown in Fig. 2. The Au coating greatly improved the anodic photocurrents and a broad peak was obtained at around 550 nm. This electrode showed an extinction peak at 580 nm [Fig. 1(c)]. There is a possibility that the influence of the high refractive index substrate is weakened by the Au coating and therefore the distal mode is suppressed. The photocurrent response at the peak was about 20 times higher than that without the Au coating. We also coated the Au layer directly on and examined its photocurrent responses for comparison. This electrode also exhibited photocurrent responses that increased gradually with increasing wavelength from about 500 nm. The extinction spectrum of the Au film showed a similar trend [Fig. 1(c)]. In general, a thin Au film prepared by evaporation consists of interconnected fine Au NPs, and hence it exhibits not only the propagating surface plasmon resonance, but also LSPR.33 Therefore, the present Au film also injects electrons into and exhibits photocurrents based on PICS [Fig. 3(b)]. Actually, nanoparticulate structures of 10- to 20-nm size were observed by SEM [Fig. 1(b)]. With reference to the response of this electrode, the introduction of Ag NCs under the Au thin film enhanced the photocurrents by approximately five times. 3.3.Mechanisms of the Photocurrent EnhancementThere are two possible reasons that the response from the NC/Au electrode is much higher than those from the NC and electrodes. One of them is “PICS with accelerated electron transfer” shown in Figs. 3(c) and 4(a), in which electron injection from the Ag NC or Ag@Au core-shell NC to is promoted by good electronic contact between the Au film and . The other one is “PICS enhanced by the nanoantenna effect” shown in Figs. 3(d) and 4(b), in which the electron injection from Au to is enhanced by optical near field generated by the Ag NC; the Ag NC absorbs light and generates optical near field in its vicinity, and then the Au film is efficiently excited by the near field (i.e., the nanoantenna effect23–25) and causes PICS. In both cases, the current would be increased by increasing the amount of Ag NCs.34 Fig. 4Possible mechanisms of (a) the PICS with accelerated electron transfer and (b) PICS enhanced by the nanoantenna effect. 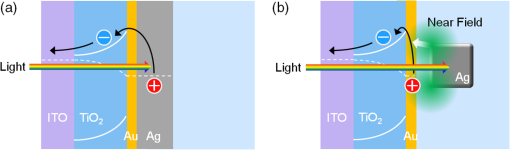 In order to obtain further information about the mechanisms of photocurrent enhancement, we prepared another type of electrode by drop-casting the Ag NCs onto the Au thin film coated directly on . The photocurrent action spectrum of the obtained NC electrode was similar to that of the NC/Au electrode (Fig. 2), and a broad peak was observed at around 535 nm. The photocurrent enhancement factors at the peak wavelength are 23 and 5 with reference to the NC and the electrodes, respectively. These photocurrent enhancements can also be explained in terms of both of the two possible mechanisms, namely the PICS with accelerated electron transfer and PICS enhanced by the nanoantenna effect. However, it is more likely that the Au thin film is excited by the optical near field of the NC proximal mode (i.e., the nanoantenna effect) because the PVP may also block the electron transfer from Ag NCs to the Au film. 4.ConclusionsPhotocurrent responses based on PICS from the NC/Au and the NC electrodes are much higher than those from the NC and electrodes. These enhancements are likely due to PICS with accelerated electron transfer or PICS enhanced by the nanoantenna effect. These results would provide an easier way to improve PICS efficiency of photovoltaics, photocatalysis, and other systems with commercially available or chemically synthesized plasmonic NPs with protecting layers. AcknowledgmentsThe authors are grateful to Gyu Min Kim for his help with immobilization of the NPs. This work was supported in part by a Grant-in-Aid for Scientific Research (Nos. 16H02082 and 16K14017) from the Japan Society for the Promotion of Science. ReferencesH. A. Atwater and A. Polman,
“Plasmonics for improved photovoltaic devices,”
Nat. Mater., 9 205
–213
(2010). http://dx.doi.org/10.1038/nmat2629 NANOHLNMAACR 2040-33641476-1122 Google Scholar
Y. Tian and T. Tatsuma,
“Mechanisms and applications of plasmon-induced charge separation at films loaded with gold nanoparticles,”
J. Am. Chem. Soc., 127 7632
–7637
(2005). http://dx.doi.org/10.1021/ja042192u JACSAT 0002-7863 Google Scholar
N. Sakai et al.,
“Plasmon resonance-based generation of cathodic photocurrent at electrodeposited gold nanoparticles coated with films,”
ChemPhysChem, 10 766
–769
(2009). http://dx.doi.org/10.1002/cphc.200800704 CPCHFT 1439-4235 Google Scholar
Y. Tian and T. Tatsuma,
“Plasmon-induced photoelectrochemistry at metal nanoparticles supported on nanoporous ,”
Chem. Commun., 16 1810
–1811
(2004). http://dx.doi.org/10.1039/B405061D Google Scholar
Y. Takahashia and T. Tatsuma,
“Electrodeposition of thermally stable gold and silver nanoparticle ensembles through a thin alumina nanomask,”
Nanoscale, 2 1494
–1499
(2010). http://dx.doi.org/10.1039/C0NR00230E NANOHL 2040-3364 Google Scholar
T. Yamaguchi et al.,
“Photoelectrochemical responses from polymer-coated plasmonic copper nanoparticles on ,”
Chem. Lett., 41 1340
–1342
(2012). http://dx.doi.org/10.1246/cl.2012.1340 CMLTAG 0366-7022 Google Scholar
E. Kazuma and T. Tatsuma,
“In-situ nanoimaging of photoinduced charge separation at the plasmonic Au nanoparticle- interface,”
Adv. Mater. Interfaces, 1 1400066
(2014). http://dx.doi.org/10.1002/admi.201400066 Google Scholar
K. Yu, N. Sakai and T. Tatsuma,
“Plasmon resonance-based solid-state photovoltaic devices,”
Electrochemistry, 76 161
–164
(2008). http://dx.doi.org/10.5796/electrochemistry.76.161 ECHMBU 0305-9979 Google Scholar
Y. Takahashi and T. Tatsuma,
“Solid state photovoltaic cells based on localized surface plasmon-induced charge separation,”
Appl. Phys. Lett., 99 182110
(2011). http://dx.doi.org/10.1063/1.3659476 APPLAB 0003-6951 Google Scholar
Y. K. Lee et al.,
“Surface plasmon-driven hot electron flow probed with metal-semiconductor nanodiodes,”
Nano Lett., 11 4251
–4255
(2011). http://dx.doi.org/10.1021/nl2022459 NALEFD 1530-6984 Google Scholar
S. Mubeen et al.,
“Plasmonic photosensitization of a wide band gap semiconductor: converting plasmons to charge carriers,”
Nano Lett., 11 5548
–5552
(2011). http://dx.doi.org/10.1021/nl203457v NALEFD 1530-6984 Google Scholar
P. Reineck et al.,
“A solid‐state plasmonic solar cell via metal nanoparticle self‐assembly,”
Adv. Mater., 24 4750
–4755
(2012). http://dx.doi.org/10.1002/adma.201200994 ADVMEW 0935-9648 Google Scholar
E. Kowalska, R. Abe and B. Ohtani,
“Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: action spectrum analysis,”
Chem. Commun., 241
–243
(2009). http://dx.doi.org/10.1039/B815679D Google Scholar
A. Tanaka, K. Hashimoto and H. Kominami,
“Preparation of exhibiting strong surface plasmon resonance effective for selective or chemoselective oxidation of alcohols to aldehydes or ketones in aqueous suspensions under irradiation by green light,”
J. Am. Chem. Soc., 134 14526
–14533
(2012). http://dx.doi.org/10.1021/ja305225s JACSAT 0002-7863 Google Scholar
Y. Ohko et al.,
“Multicolor photochromism of films loaded with Ag nanoparticles,”
Nat. Mater., 2 29
–31
(2003). http://dx.doi.org/10.1038/nmat796 NMAACR 1476-1122 Google Scholar
E. Kazuma and T. Tatsuma,
“Photoinduced reversible changes in morphology of plasmonic Ag nanorods on and application to versatile photochromism,”
Chem. Commun., 48 1733
–1735
(2012). http://dx.doi.org/10.1039/C2CC16589A Google Scholar
I. Tanabe and T. Tatsuma,
“Plasmonic manipulation of color and morphology of single silver nanospheres,”
Nano Lett., 12 5418
–5421
(2012). http://dx.doi.org/10.1021/nl302919n NALEFD 1530-6984 Google Scholar
T. Tatsuma, K. Takada and T. Miyazaki,
“UV light-induced swelling and visible light-induced shrinking of a -containing redox gel,”
Adv. Mater., 19 1249
–1251
(2007). http://dx.doi.org/10.1002/adma.200602386 ADVMEW 0935-9648 Google Scholar
T. Tatsuma et al.,
“Direct output of electrical signals from LSPR sensors on the basis of plasmon-induced charge separation,”
Chem. Commun., 51 6100
–6103
(2015). http://dx.doi.org/10.1039/C5CC01020A Google Scholar
K. Yu, Y. Tian and T. Tatsuma,
“Size effects of gold nanoparticles on plasmon-induced photocurrents of gold- nanocomposites,”
Phys. Chem. Chem. Phys., 8 5417
–5420
(2006). http://dx.doi.org/10.1039/B610720F PPCPFQ 1463-9076 Google Scholar
L. Wu, H. Nishi and T. Tatsuma,
“Plasmon-induced charge separation at two-dimensional gold semishell arrays on colloidal crystals,”
APL Mater., 3 104406
(2015). http://dx.doi.org/10.1063/1.492293510.1063/1.4922935 Google Scholar
Y. Katagi, E. Kazuma and T. Tatsuma,
“Photoelectrochemical synthesis, optical properties and plasmon-induced charge separation behaviour of gold nanodumbbells on ,”
Nanoscale, 6 14543
–14548
(2014). http://dx.doi.org/10.1039/C4NR05282J NANOHL 2040-3364 Google Scholar
S.-W. Baek et al.,
“Au@Ag core-shell nanocubes for efficient plasmonic light scattering effect in low bandgap organic solar cells,”
ACS Nano, 8 3302
–3312
(2014). http://dx.doi.org/10.1021/nn500222q ANCAC3 1936-0851 Google Scholar
T. Kawawaki et al.,
“Efficiency enhancement of PbS quantum dot/ZnO nanowire bulk-heterojunction solar cells by plasmonic silver nanocubes,”
ACS Nano, 9 4165
–4172
(2015). http://dx.doi.org/10.1021/acsnano.5b00321 ANCAC3 1936-0851 Google Scholar
K. Leonard et al.,
“Enhanced photoelectrochemical response of polythiophene photoelectrodes with controlled arrays of silver nanocubes,”
J. Phys. Chem. C, 119 8829
–8837
(2015). http://dx.doi.org/10.1021/jp5114366 JPCCCK 1932-7447 Google Scholar
L. J. Sherry et al.,
“Localized surface plasmon resonance spectroscopy of single silver nanocubes,”
Nano Lett., 5 2034
–2038
(2005). http://dx.doi.org/10.1021/nl0515753 NALEFD 1530-6984 Google Scholar
A. Moreau et al.,
“Controlled-reflectance surfaces with film-coupled colloidal nanoantennas,”
Nature, 492 86
–89
(2012). http://dx.doi.org/10.1038/nature11615 Google Scholar
K. Saito and T. Tatsuma,
“Asymmetric three-way plasmonic color routers,”
Adv. Opt. Mater., 3 883
–887
(2015). http://dx.doi.org/10.1002/adom.201500111 2195-1071 Google Scholar
K. Saito and T. Tatsuma,
“A transparent projection screen based on plasmonic Ag nanocubes,”
Nanoscale, 7 20365
–20368
(2015). http://dx.doi.org/10.1039/C5NR06766A Google Scholar
S. H. Im et al.,
“Large-scale synthesis of silver nanocubes: the role of HCl in promoting cube perfection and monodispersity,”
Angew. Chem., Int. Ed., 117 2192
–2195
(2005). http://dx.doi.org/10.1002/(ISSN)1521-3757 Google Scholar
M. Luo et al.,
“Facile removal of polyvinylpyrrolidone (PVP) adsorbates from Pt alloy nanoparticles,”
J. Mater. Chem. A, 3 2770
–2775
(2015). http://dx.doi.org/10.1039/C4TA05250A Google Scholar
Z. Niu and Y. Li,
“Removal and utilization of capping agents in nanocatalysis,”
Chem. Mater., 26 72
–83
(2014). http://dx.doi.org/10.1021/cm4022479 CMATEX 0897-4756 Google Scholar
R. Gupta, M. J. Dyer and W. A. Weimer,
“Preparation and characterization of surface plasmon resonance tunable gold and silver films,”
J. Appl. Phys., 92 5264
–5270
(2002). http://dx.doi.org/10.1063/1.1511275 JAPIAU 0021-8979 Google Scholar
T. Kawawaki, Y. Takahashi and T. Tatsuma,
“Enhancement of PbS quantum dot-sensitized photocurrents by plasmonic gold nanoparticles,”
J. Phys. Chem. C, 117 5901
–5907
(2013). http://dx.doi.org/10.1039/C3CP53625D JPCCCK 1932-7447 Google Scholar
BiographyKazutaka Akiyoshi is a PhD candidate in University of Tokyo, Japan. He received his BSc degree from Tokyo University of Science in 2014 and MSc from University of Tokyo in 2016. His research focuses mainly on development of photoelectrochemical devices based on plasmonic nanoparticles. Koichiro Saito is a PhD candidate in University of Tokyo, Japan. He received his BSc in 2013 and MSc in 2015 degrees from University of Tokyo. His research focuses mainly on fabrication and photo-manipulation of plasmonic nanomaterials and application to photonic materials and devices. Tetsu Tatsuma is a professor at University of Tokyo, Japan. He received his BSc in 1988, MSc in 1990, and PhD in 1993 degrees in electrochemistry from University of Tokyo. His current research interests include electrochemistry and plasmonics, in particular mechanistic analysis of plasmon-induced charge separation and its application to photovoltaics, photocatalysis, photochromism, sensors, and actuators. |